History, Taxonomy, Morphology and Physiology of Bacteria and Microbial Pathogenicity Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
| History of Microbiology |

|
| Bacterial Taxonomy |

|
| Microscopy |

|
| Staining Techniques |

|
| Morphology of Bacteria |

|
| Physiology of Bacteria (Bacterial Growth and Nutrition) |

|
| Microbial Pathogenicity |

|
History of Microbiology

Louis Pasteur
Louis Pasteur is often called the father of microbiology because of his important work in the field. He came up with the ideas of fermentation to help keep food safe, introduced methods for sterilization, and invented essential equipment like the steam sterilizer, hot air oven, and autoclave. He is famous for his process of pasteurization to make milk safe and played a vital role in creating vaccines for diseases such as anthrax, fowl cholera, and rabies.
Pasteur challenged the old belief in spontaneous generation and put forward the germ theory of disease, which suggests that diseases are caused by tiny organisms in the air, not by bad air or fumes. He also used nutrient broth to grow microorganisms and established the Pasteur Institute in Paris.
Louis Pasteur proposed:
- Principles of fermentation
- Pasteurization of milk
- Sterilization techniques
- Germ theory of disease
Robert Koch
Robert Koch made important advancements in microbiology, including:
- Use of Solid Media: Koch utilized solid media to culture bacteria, introducing agar as a solidifying agent, inspired by Eilshemius Hesse, the wife of one of his assistants.
- Pure Culture Isolation: He developed techniques for isolating bacteria in pure culture.
- Hanging Drop Method: Koch described the hanging drop method to assess bacterial motility.
- Bacterial Discoveries: He discovered crucial bacteria such as anthrax bacilli, tubercle bacilli, and cholera bacilli.
- Staining Techniques: Koch introduced staining methods using aniline dye.
- Koch’s Phenomenon: He observed that guinea pigs previously infected with tubercle bacillus exhibited a hypersensitivity reaction when injected with the bacilli or its protein.
Koch's Postulates
- Mycobacterium leprae, Treponema pallidum, and Neisseria gonorrhoeae are exceptions to Koch's postulates.
- A microorganism is considered the cause of an infectious disease if:
- It is found in the lesions of the disease.
- It can be isolated in pure culture from the lesions.
- The same disease occurs when the isolated microorganism is inoculated into a suitable laboratory animal.
- It can be re-isolated in pure culture from the lesions in the experimental animals.
- An additional criterion is the detection of antibodies to the causative organism in the patient’s serum.
- These postulates may not apply to human diseases, as some bacteria do not meet all four criteria:
- Mycobacterium leprae and Treponema pallidum cannot be grown in vitro but can be maintained in animals.
- Neisseria gonorrhoeae cannot be studied in animals, but the bacteria can be grown in vitro.
- Molecular Koch’s Postulates: Stanley Falkow proposed that the gene responsible for virulence should meet all of Koch’s postulate criteria instead of the microorganism itself.
Paul Ehrlich
Paul Ehrlich was the pioneer in identifying the acid-fast characteristic of the tubercle bacillus.
He also developed methods for staining tissues and blood cells.
Significant Contributions:
- A. van Leeuwenhoek. Innovator of the simple microscope
- Ernst Ruska. Developer of the electron microscope
- E. Jenner. Creator of the smallpox vaccine
- Kary B. Mullis. Pioneer of PCR (Polymerase Chain Reaction)
Ehrlich proposed the interaction between toxin and antitoxin, known as the Ehrlich phenomenon, and introduced methods for standardising these substances.
He also put forward the side chain theory for antibody production.
Furthermore, Ehrlich discovered salvarsan, an arsenical compound used as a magic bullet for treating syphilis, earning him the title of the father of chemotherapy.
In honour of his contributions, the bacterium Ehrlichia was named after him.
Other Significant Figures in Microbiology
- Antonie Philips van Leeuwenhoek: He invented the single-lens microscope and referred to tiny organisms as "little animalcules."
- Edward Jenner: He developed the first vaccine for smallpox using the cowpox virus.
- Joseph Lister: Known as the pioneer of antiseptic surgery, he employed carbolic acid during surgical procedures.
- Hans Christian Gram: He is recognized for creating the "Gram stain."
- Ernst Ruska: He was the founder of the electron microscope.
- Alexander Fleming: He is famous for discovering the antibiotic penicillin in 1928.
- Elie Metchnikoff: He introduced the concept of phagocytosis and coined the term phagocytes.
- Kleinberger: He identified L forms of bacteria.
- Barbara McClintock: She explained the phenomenon of transposons.
- Walter Gilbert and Frederick Sanger: They were the first to develop a method for DNA sequencing in 1977.
- Kary B. Mullis: He is known for discovering the polymerase chain reaction (PCR).
Discovery of Significant Microorganisms
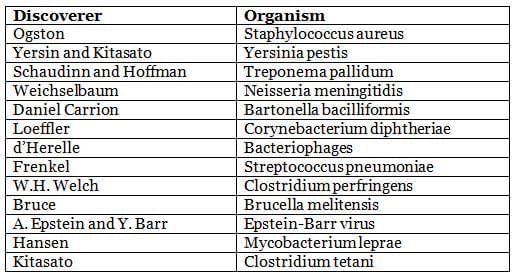
Common Names of Bacteria Derived from Discoverers
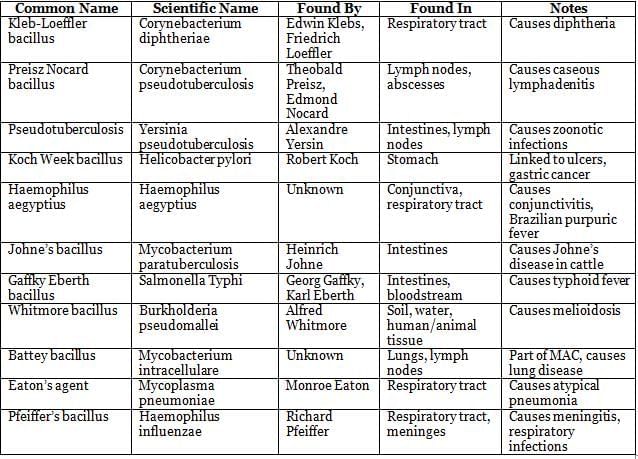
Bacterial Taxonomy
In 1998, Cavalier-Smith proposed a six-kingdom classification system, which was a significant step forward in taxonomic classification. However, more recent models have introduced the concept of domains. Cavalier-Smith's system divides organisms into six kingdoms: Bacteria, Protozoa, Chromista, Plantae, Fungi, and Animalia.
Principles Used for Bacterial Classification
- Phylogenetic Classification: This method organizes organisms into a hierarchical system that resembles a branching tree. Each branch point (node) in the tree represents a characteristic or trait used to differentiate organisms.
- Adansonian (or Phonetic) Classification: Proposed by Michel Adanson, this approach treats all characteristics equally to avoid bias in classification. It is often used in numeric taxonomy.
- Molecular Classification: This method focuses on the genetic relatedness of organisms, specifically examining the guanine (G) and cytosine (C) content in their DNA.
Microscopy
Microscopy is a technique used to observe objects that are too small to be seen with the naked eye. A good microscope should have three essential properties:
Properties of a Good Microscope
- Good resolution: This refers to the ability to see closely placed objects as distinct entities. The resolution power varies among different types of microscopes:
- Unaided human eye: about 0.2 mm (200 µm)
- Light microscope: about 0.2 µm for high-quality models, though it can vary by type.
- Electron microscope: about 0.5 nm
- Good contrast: Contrast is crucial for identifying specific structures within a specimen. Staining techniques are commonly used to improve contrast by highlighting particular features.
- Good magnification: Magnification is the process of enlarging the appearance of an object, and it is achieved through the use of convex lenses in a microscope.
Resolution is influenced by the refractive index, which is higher for oil than for air. This is why oil immersion lenses are used in microscopy to enhance resolution.
Bright-Field or Light Microscope
The bright-field or light microscope produces an image where the specimen appears dark against a lighter background.
Darkfield Microscope
- Principle:. darkfield microscope makes the object appear bright on a dark background using a specialized darkfield condenser.
- Applications: This type of microscope is useful for identifying living, unstained cells and thin bacteria, such as spirochetes, which are challenging to observe with standard light microscopy.
Phase Contrast Microscope
- The phase contrast microscope is designed to observe living cells by enhancing the contrast between the cells and the surrounding water.
- It converts subtle variations in refractive index and cell density into significant differences in light intensity, making it easier to see the cells.
- This type of microscope is beneficial for studying:
- Microbial motility: Understanding how microorganisms move.
- Determining the shape of living cells: Observing the various shapes of cells while they are alive.
- Detecting bacterial components: Identifying structures within bacteria, such as endospores and inclusion bodies.
Fluorescence Microscope
Principle: Fluorescent dyes emit visible light when exposed to ultraviolet (UV) rays.
Applications: The epifluorescence microscope is used for:
- Autofluorescence: Observing substances like Cyclospora under a UV lamp.
- Microbes Coated with Fluorescent Dye: Using Acridine orange to stain malaria parasites in the QBC method and Auramine phenol for M. tuberculosis.
- Immunofluorescence: Employing antibodies tagged with fluorescent dyes to detect cell surface antigens or antibodies attached to these antigens, including direct IF, indirect IF, and flow cytometry.
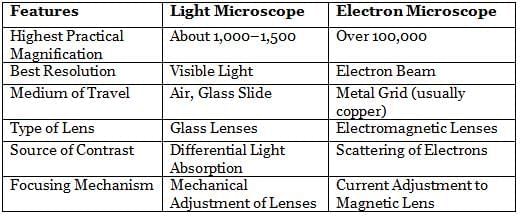
Electron Microscope
The electron microscope, invented by Ernst Ruska in 1931, differs from the light microscope in various aspects. There are two main types of electron microscopes:
- Transmission Electron Microscope (TEM). This type examines the internal structure of specimens, offering a resolution of 0.5 nm and providing a two-dimensional view.
- Scanning Electron Microscope (SEM). SEM focuses on the surfaces of specimens, with a resolution of 7 nm and providing a three-dimensional view.
Bacterial Classification
- Phylogenetic Classification. This method is based on weighted characteristics of bacteria, considering the evolutionary relationships between different species.
- Adansonian (or Phonetic) Classification. In this approach, equal weight is given to every characteristic of the bacteria, regardless of its importance, to classify them.
- Molecular Classification. This method relies on the genetic relatedness of bacteria, using molecular data to determine their relationships.
Resolution Power
- Unaided Human Eye: 0.2 mm
- Light Microscope: 0.2 µm
- Electron Microscope: 0.5 nm
Numerical Aperture. n sin α (where n is the refractive index of the medium and α is half the angular aperture of the objective).
Resolving Power. 0.61 × wavelength/Numerical aperture.
Type of Light Used in Microscopes
- Transmitted Light: Used in bright field microscopy.
- Reflected Light: Used in dark field microscopy.
- Polarized Light: Used in differential interference contrast microscopy.
Applications of Fluorescence Microscope
- Auto fluorescence: Detecting organisms like Cyclospora that naturally fluoresce.
- Microbes Coated with Fluorescent Dye: Using techniques like QBC for detecting Plasmodium and Auramine phenol for identifying M. tuberculosis.
- Immunofluorescence: Tagging antibodies or antigens with fluorescent dyes to visualize specific proteins or pathogens.
Electron Microscope
- Magnification: Over 100,000 times.
- Resolution: 0.5 nm.
- Radiation Source: Electron beam.
- Medium: High vacuum.
- Specimen Preparation: Mounted on a metal grid.
- Electromagnet Lens used for focusing.
Differences between Light Microscope and Electron Microscope
Principle of Transmission Electron Microscopy
- Specimen preparation: Cells go through various steps to create very thin specimens, measuring between 20 to 100 nm thick.
- Fixation: Cells are stabilised using glutaraldehyde or osmium tetroxide.
- Dehydration: Specimens are dehydrated using organic solvents, such as acetone or ethanol.
- Embedding: Specimens are embedded in a plastic polymer, which is then hardened to form a solid block. Complete dehydration is essential as most plastic polymers are water insoluble.
- Slicing: Specimens are sliced into thin sections with an ultramicrotome and are placed on a metal slide, typically copper.
- Freeze-etching technique: This is an alternative method for preparing specimens to view the internal organelles. Cells are rapidly frozen, warmed, fractured to expose the organelles, subjected to sublimation, and finally shadowed with a coating of platinum and carbon.
Measures to increase the contrast of EM include:
- Staining: This involves using solutions of heavy metal salts like lead citrate and uranyl acetate.
- Negative staining: Heavy metals, such as phosphotungstic acid or uranyl acetate, are used for this purpose.
- Shadowing: Specimens are coated with a thin film of a heavy metal, like platinum, at a 45° angle, allowing the metal to contact the microorganism from only one side.
Staining Techniques
Staining is a crucial step in microbiology as it creates a color contrast that enhances the visibility of the specimen. Before staining, the smear must be fixed to the slide to preserve the sample.
Fixation Methods:
- Heat Fixation: Commonly used for bacterial smears by gently heating an air-dried film of bacteria over a flame.
- Chemical Fixation:Involves the use of various chemicals such as:
- Ethanol
- Acetic Acid
- Mercuric Chloride
- Formaldehyde
- Methanol
- Glutaraldehyde
Staining Techniques Used in Microbiology
- Simple Stain: Involves the use of basic dyes like methylene blue or basic fuchsin, which provide color contrast but stain all bacteria in a smear the same color.
- Negative Staining: Techniques such as India ink or nigrosin are used to stain the background black, allowing unstained bacteria to stand out. This method is particularly useful for demonstrating bacterial capsules that do not absorb simple stains.
- Impregnation Methods: Such as silver staining, are used to show thin structures like bacterial flagella and spirochetes.
- Differential Stain:Gram Stain:Differentiates bacteria into Gram-positive (appear violet) and Gram-negative (appear pink) based on their cell wall characteristics.
- Primary stain with crystal violet (or gentian violet or methyl violet) for one minute.
- Mordant with Gram’s iodine for one minute.
- Decolorization with acetone (for 1–2 seconds) or ethanol (20–30 seconds) followed by immediate wash. This step removes the primary stain from Gram-negative bacteria while Gram-positive bacteria retain it.
- Counter stain with safranin or diluted carbol fuchsin for 30 seconds.
- Acid-Fast Stain: Used for acid-fast organisms that resist decolorization by mineral acids due to the presence of mycolic acids in their cell walls.
- Albert Stain: Differentiates bacteria with metachromatic granules (e.g., Corynebacterium diphtheriae) from those without.
Other Special Staining Methods:
- Spore Staining: Involves using sulfuric acid during the decolorization step and Malachite green (Schaeffer and Fulton method modified by Ashby); however, phase contrast microscopy of unstained wet films is considered the best method.
- Lipid Staining: Lipids are stained with Sudan Black.
- Carbohydrate Staining (Glycogen): Stained with Iodine.
- Flagellar Staining: Utilizes tannic acid (Leifson method) to stain flagella.
Microscopy of Bacteria in Living State:
- Unstained (wet) preparations: This method is used to check for bacterial motility, such as in hanging drop and wet mount preparations.
- It is also used for demonstrating spirochetes, such as in dark field or phase contrast microscopy.
- Vital stains are used to differentiate living cells from dead ones.
- Supravital staining involves staining living cells after they have been removed from an organism.
- Intravital staining is performed by injecting the stain into the body.
- Examples of vital stains include eosin, propidium iodide, trypan blue, erythrosine, and neutral red.
- Spore staining. Acid-fast staining typically uses carbol fuchsin and is not defined by sulfuric acid concentration; the Schaeffer and Fulton method is also used.
- Carbohydrate staining is done using iodine stain.
- Flagellar stain. Utilizes tannic acid (Leifson method).
Morphology of Bacteria
Prokaryotes vs. Eukaryotes
- Prokaryotes. Include bacteria and blue-green algae.
- Eukaryotes. Include fungi, parasites, other algae, plants, and animals.
- Nucleus. In prokaryotes, the nucleus is diffuse, while in eukaryotes, it is well-defined.
- Nuclear membrane. Absent in prokaryotes and present in eukaryotes.
- Nucleolus. Composed of ribonucleoprotein in prokaryotes.
- Cell division. Prokaryotes divide by binary fission, while eukaryotes undergo mitosis or meiosis.
- Chromosome. Prokaryotes have one circular chromosome, whereas eukaryotes have many linear chromosomes.
- Extra chromosomal DNA. Found in plasmids in prokaryotes and in mitochondria in eukaryotes.
- Sterols in cell membrane. Absent in prokaryotes, except in mycoplasma.
- Cellular organelles. Absent in prokaryotes, except for ribosomes.
- Ribosome. 70s in prokaryotes and 80s in eukaryotes.
- Site of respiration. Mesosome in prokaryotes and mitochondria in eukaryotes.
- Pinocytosis. Present in eukaryotes.
Shape of Bacteria and Their Gram Staining Property
1. Gram-Positive Cocci:
- Cluster: Staphylococcus
- Chain: Streptococcus
- Pairs, Lanceolate Shaped: Pneumococcus
- Tetrads: Micrococcus
- Octate: Sarcina
- Pair, Spectacle Eyed Shaped: Enterococcus
2. Gram-Positive Bacilli:
- Chain: Bacillus anthracis (Bamboo Stick Appearance)
- Chinese Letter Arrangement: C. diphtheriae
- Pallisade Arrangement: Other Corynebacterium species
- Branching GPB: Actinomycetes
3. Gram-Negative Cocci:
- Pairs, Lens Shaped: Meningococcus
- Pairs, Kidney Shaped: Gonococcus
4. Gram-Negative Bacilli:
- Pleomorphic: Haemophilus, Proteus
- Thumbprint-Like Appearance: Bordetella pertussis
- Curved: Campylobacter (Gull-Wing Shaped) and Helicobacter
- Spirally Coiled, Rigid: Spirillum
- Spirally Coiled, Flexible: Spirochetes
- Comma Shaped: Vibrio cholerae
- Streptobacillus: Various species
Bacterial Cell Wall
The cell wall is a tough and rigid layer that encloses the bacterium. It provides protection and helps maintain the bacterium's shape. The cell wall also contains components like lipopolysaccharides (LPS) that can trigger immune responses and act as virulence factors.
The main material of the cell wall is peptidoglycan, which gives it strength. Peptidoglycan is made up of alternating units of N-acetyl muramic acid (NAM) and N-acetyl glucosamine (NAG), linked together by tetrapeptide side chains and pentaglycine bridges.
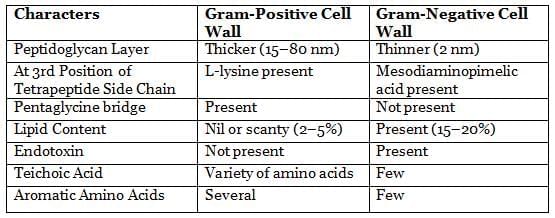
Gram-positive bacteria have a thicker peptidoglycan layer and contain teichoic acid.
Gram-negative bacteria have a thinner peptidoglycan layer and additional features such as:
- Outer membrane
- Lipopolysaccharide (LPS), which consists of:
- Lipid A (which acts as an endotoxin)
- Core polysaccharide
- O side chain
Differences Between Gram-Positive and Gram-Negative Cell Walls
Other Parts of Bacterial Cells
Intracytoplasmic Inclusions
Intracytoplasmic inclusions refer to the storage sites of nutrients in bacteria, which are formed during times of food scarcity.
- Organic inclusion bodies: These include storage materials such as glycogen granules and poly-hydroxyl butyrate granules.
- Inorganic inclusion bodies: These include:
- Polymetaphosphate: Also known as volutin or metachromatic granules, these are found in bacteria like C. diphtheriae .
- Sulfur granules: These are located in bacteria such as Actinomyces .
Nucleoid
- Bacteria do not have a true nucleus. Instead, their genetic material is located in a region called the nucleoid, which has an irregular shape.
- The nucleoid does not have a nuclear membrane or a nucleolus, and it also lacks basic proteins.
- Inside the nucleoid, bacteria possess a single haploid chromosome made of supercoiled circular double-stranded DNA. However, some species like Vibrio may have two chromosomes.
- Before a bacterial cell divides, it replicates its DNA through a process known as binary fission.
- The nucleoid can be seen using electron microscopy or by staining it with the Feulgen stain.
- In addition to the chromosomal DNA, bacteria also have extra-chromosomal DNA called plasmids.
Capsule and Slime Layer
Some bacteria possess a layer of sticky, jelly-like material outside their cell wall called glycocalyx. This layer can be either well-organized, known as a capsule, or loosely arranged, referred to as a slime layer. Some bacteria, such as Streptococcus salivarius, may have both a capsule and a slime layer.
Gram Positive cell wall:
- Thicker Peptidoglycan
- Absent: LPS, Aromatic AA, and lipids
- Present: Teichoic acid
Capsulated bacteria:
- Haemophilus influenzae
- Klebsiella pneumoniae
- Pseudomonas aeruginosa
Capsulated fungus:
- Cryptococcus
Composition:
- Polysaccharide
- Polypeptide (glutamate)
- Streptococcus pyogenes (some strains)
- Hyaluronic acid
- Bacteroides fragilis
- Cryptococcus neoformans
The capsule serves several crucial functions:
- It helps prevent phagocytosis and complement-mediated lysis.
- It aids in biofilm formation, facilitating attachment to damaged tissues and plastic surfaces, such as medical devices.
- It provides protection and assists in nutrient absorption for the bacteria.
- Capsules are utilized in vaccines, including those for Pneumococcus, Meningococcus, Haemophilus influenzae serotype-b, and S. Typhi Vi Vaccine.
Demonstrating the capsule can be achieved through:
- Negative staining with India ink and nigrosin stain. The capsule appears as a clear halo around the bacteria, while the bacteria and background are stained black.
Types of motility:
- Tumbling: Listeria
- Gliding: Mycoplasma
- Stately: Clostridium
- Darting: Vibrio, Campylobacter
- Swarming: Proteus, C. tetani
- Corkscrew, lashing, flexion extension: Spirochete
M’Faydean capsule stain method for Bacillus anthracis using polychrome methylene blue stain.
Serological test: Capsular material is antigenic and can be demonstrated by mixing it with specific anticapsular serum:
- Quellung reaction for Streptococcus pneumoniae
- Latex agglutination test using specific anticapsular antibodies coated on latex.
Flagella are thread-like structures that extend from the cell wall and are made of protein subunits called flagellin. They consist of three parts: filament, hook, and basal body.
- Size: They are typically 5–20 µm long and 0.01–0.02 µm thick.
- Flagella are used for movement, providing motility to the bacteria.
Arrangement of flagella:
- Monotrichous (single polar flagellum), e.g., Vibrio cholerae, Pseudomonas, and Campylobacter.
- Lophotrichous (multiple polar flagella), e.g., Spirillum.
- Peritrichous (over the entire cell surface), e.g., Salmonella Typhi, Escherichia coli.
- Amphitrichous (single flagellum at both ends), e.g., Alcaligenes faecalis, Spirillum.
- (Note: Spirillum has a tuft of flagella at one or both ends (Amphi > lophotrichous).
Flagella can be demonstrated by:
- Direct observation of flagella.
- Tannic acid staining (Leifson’s method and Ryu’s method).
- Using dark ground, phase contrast, or electron microscopy.
Indirect methods can show motility:
- Cragie tube method and Hanging drop method.
- Semi-solid medium, e.g., mannitol motility medium.
Types of Motility in Bacteria
- Bacteria Shown: Various types of motility displayed by different bacteria.
- Tumbling Motility:. type of movement characterized by a tumbling motion.
- Gliding Motility:. smooth, gliding type of movement.
- Stately Motility:. type of movement that is steady and deliberate.
- Darting Motility:. quick, darting type of movement.
- Vibrio cholerae and Campylobacter: Bacteria that exhibit specific types of motility.
- Swarming Motility:. type of movement where bacteria swarm together.
- Proteus and Clostridium tetani: Examples of bacteria that exhibit swarming motility.
Fimbriae or Pili
- Many Gram-negative and some Gram-positive bacteria possess short, thin, hair-like appendages known as fimbriae or pili. These structures are thinner than flagella and are not used for movement. Fimbriae are approximately 0.5 µm long and 10 nm thick.
- Pili are primarily made of a protein called pilin.
- Fimbriae and pili can trigger an immune response, although antibodies against fimbrial antigens do not provide protection.
- Function: Fimbriae play a crucial role in helping bacteria adhere to surfaces, which enhances their ability to cause disease. Some fimbriae, known as sex pili, facilitate the transfer of genes between bacteria. Fimbriae are not associated with movement and can be found in both motile and non-motile bacteria.
- Common types of pili include:
- Sex or F (fertility) pili: These pili assist in bacterial conjugation and are found in bacteria such as Gonococcus.
- Col I (colicin) pili: These pili are involved in the production of colicins, which are toxins produced by certain bacteria.
- Detection of Fimbriae:
- Direct Observation: Fimbriae can be directly observed using electron microscopy.
- Indirect Detection Methods:
- Hemagglutination: Many fimbriated bacteria, such as Escherichia coli and Klebsiella, have the ability to agglutinate red blood cells. This reaction can be specifically inhibited by D-mannose.
- Some aerobic fimbriated bacteria form a thin layer on the surface of broth cultures, which can be used for indirect detection.
Atypical Forms of Bacteria
- Involution Forms: These are abnormal and swollen types of bacteria, like Gonococci and Yersinia pestis, seen in older cultures.
- Pleomorphic Bacteria: These bacteria can exhibit different shapes and sizes in individual cells, such as Proteus and Yersinia pestis.
- L Forms or Cell Wall-Deficient Forms: When bacteria lose their cell wall, they become round and are referred to as L forms.
- These L forms were discovered by E. Klieneberger while studying Streptobacillus moniliformis.
- The name "L forms" comes from the Lister Institute in London, where they were first identified.
- L forms may contribute to the persistence of conditions like pyelonephritis and other chronic infections.
- In Gram-positive bacteria, L forms are known as Protoplasts, while in Gram-negative bacteria, they are called Spheroplasts.
- Mycoplasma are unique because they lack a true cell wall. Instead of a peptidoglycan layer, they have sterols. Some researchers propose that Mycoplasma might be stable L forms of unknown parent bacteria, although this idea is debated among scientists.
Bacterial Spores
- Spores represent a highly resilient dormant phase of bacteria that occurs when environmental conditions become adverse, particularly due to a scarcity of nutrients.
- The composition of a spore consists of multiple layers, arranged from the innermost to the outermost as follows: core. cortex. coat. exosporium.
- Due to their exceptional resistance, only a limited number of sterilization methods are capable of effectively eliminating spores.
- Spores are frequently employed to verify the adequacy of sterilization processes.
- Spores of Geobacillus stearothermophilus are utilized to monitor sterilization in autoclaves.
- Spores from non-toxigenic strains of Clostridium sporogenes serve as a control for hot air ovens.
- Spores of Bacillus anthracis gained notoriety when used in the 2001 bioterrorism incident in the USA.
- Other notable examples include gonococci and Yersinia pestis.
- Spores can be identified in older bacterial cultures.
Physiology of Bacteria (Bacterial Growth and Nutrition)
1. Generation Time
- Generation Time refers to the duration required for a bacterium to divide into two daughter cells under optimal conditions.
- For Escherichia coli and most other pathogenic bacteria, the generation time is approximately 20 minutes .
- Mycobacterium tuberculosis has a longer generation time of about 14 hours .
- Mycobacterium leprae exhibits an even longer generation time, ranging from 12 to 13 days .
2. Bacterial Count
- Total Count. This method determines the overall number of bacteria in a sample, including both live and dead cells. It is done using a microscope and a counting chamber.
- Viable Count. This measures the number of living cells in the sample. There are several methods to determine viable count, including:
- Pour Plate Method. Considered the most reliable method.
- Surface Viable Count by Spreading Method .
- Surface Viable Count by Miles and Misra Method .
Bacterial Growth Curve
When bacteria are placed in a suitable liquid culture medium, they undergo a specific growth pattern that can be observed and measured over time. By counting the number of bacteria at different time intervals and plotting this data on a graph, we create a bacterial growth curve, which consists of four distinct phases:
1. Lag Phase:
- Bacterial Division: No
- Bacterial Death: Flat
- Growth Pattern: Raises
Special Features:
- Preparatory phase with accumulation of enzymes and metabolites.
- Maximum cell size attained by the end of the lag phase.
- Cells are uniformly stained, metabolically active, and small in size.
Gram variable: Bacteria exhibit variability in retaining crystal violet stain due to differences in cell wall structure.
- Production of granules, spores, exotoxins, antibiotics, bacteriocins, and involution forms.
2. Log Phase:
- Bacterial Division: Yes
- Bacterial Death: Flat
- Growth Pattern: Raises
3. Stationary Phase:
- Bacterial Division: Yes
- Bacterial Death: Yes
- Growth Pattern: Flat
4. Decline Phase:
- Bacterial Division: No
- Bacterial Death: Yes
- Growth Pattern: Falls
Factors Influencing Bacterial Growth
- Oxygen: Bacteria are classified based on their oxygen requirements:
- Obligate aerobes: Require oxygen to thrive (e.g., Pseudomonas, M. tuberculosis, Bacillus, Brucella, Nocardia ).
- Facultative anaerobes: Prefer oxygen but can survive without it (e.g., E. coli, S. aureus ).
- Obligate anaerobes: Thrive only in the absence of oxygen, which is harmful to them (e.g., Clostridium ).
- Facultative anaerobes: These are anaerobes that can also grow in oxygen (e.g. Lactobacillus ).
- Microaerophilic bacteria: Require low levels of oxygen (5–10%) for growth (e.g., Campylobacter, Helicobacter, Mycobacterium bovis ).
- Aerotolerant anaerobes: Can tolerate some oxygen but do not use it (e.g., Clostridium histolyticum ).
- Carbon Dioxide: Capnophilic bacteria require 5–10% CO2 for growth (e.g., Brucella abortus, Streptococcus pneumoniae ).
- Temperature: Bacteria are categorized based on their optimal growth temperature:
- Psychrophiles: Prefer temperatures below 20°C (e.g., saprophytic bacteria).
- Mesophiles: Thrive between 25°C and 40°C (e.g., most pathogenic bacteria).
- Thermophiles: Grow at temperatures between 55°C and 80°C (e.g., Bacillus stearothermophilus ).
- pH: Most pathogenic bacteria prefer a neutral to slightly alkaline pH. Some bacteria, like lactobacilli, can survive in acidic conditions below pH 4, while others, such as Vibrio cholerae, can grow in alkaline conditions with a pH range of 8.2 to 8.9.
- Light: Most bacteria (except for phototrophic bacteria) prefer dark conditions for growth and are sensitive to ultraviolet light. Photochromogenic mycobacteria produce pigments only when exposed to light.
- Other Factors: These include osmotic effects, mechanical and sonic stresses, moisture levels, and the effects of drying out.
Microbial Pathogenicity
Microbial pathogenicity is determined by a variety of factors, which are detailed below.
Route of Transmission of Infection
The infective dose refers to the smallest quantity of microorganisms required to initiate an infection.
- Low Infective Dose: Some pathogens can cause infection with a very small number of organisms. For example:
- Shigella: As few as 10 bacilli can cause infection.
- Cryptosporidium parvum: Requires 10 to 30 oocysts.
- Escherichia coli O157:H7: Fewer than 10 bacilli are needed.
- Entamoeba and Giardia: Require only a few cysts.
- Campylobacter jejuni: Needs 500 bacilli.
- Escherichia coli: Requires between 10 6 and 10 8 bacilli.
- Salmonella: Requires between 10 2 and 10 5 bacilli.
- Vibrio cholerae: Also requires between 10 6 and 10 8 bacilli to cause infection.
Evasion of Local Defenses
- Adhesion: Bacteria adhere using fimbriae, pili, other adhesins, and form biofilms.
- Invasion: Factors that assist in invasion include:
- Virulence Markers: Antigens or plasmids that promote invasion in Shigella.
- Enzymes:Several enzymes enhance bacterial invasion, such as:
- Hyaluronidase
- Collagenase
- Streptokinase (fibrinolysin)
- IgA proteases
- Antiphagocytic Factors: Including capsules.
- Cell Wall Proteins: Such as Protein A from Staphylococcus aureus and M protein from Streptococcus pyogenes.
- Cytotoxins: These interfere with the movement or destruction of phagocytes. For instance, S. aureus produces hemolysins and leukocidins that damage red blood cells (RBCs) and white blood cells (WBCs).
Mechanism of Intracellular Survival
- Inhibition of Phagolysosome Fusion: Observed in species like Legionella, Mycobacterium tuberculosis, and Chlamydia.
- Resistance to Lysosomal Enzymes: Found in Salmonella Typhimurium, Coxiella, Mycobacterium leprae, and Leishmania.
- Adaptation to Cytoplasmic Replication: Found in Listeria, Rickettsia, and Francisella tularensis.
- Examples of Obligate Intracellular Organisms: Bacteria: Mycobacterium leprae, Rickettsia, Chlamydia, and Coxiella.
- All Viruses
- Fungi: Pneumocystis jirovecii
- Parasites: Toxoplasma, Cryptosporidium, Plasmodium, Leishmania, Babesia, and Trypanosoma cruzi.
Differences Between Bacterial Endotoxins and Exotoxins
Endotoxins:
- Composed of lipopolysaccharides.
- Part of the cell wall of Gram-negative bacteria.
- Released upon cell damage, not by active secretion.
- Stable and resistant to destruction.
- Induces increased IL-1 and TNF.
- Causes non-specific symptoms like fever and shock.
- Lacks specific tissue affinity.
- Requires large doses to be fatal.
- Poorly antigenic and ineffective for vaccine development.
Exotoxins:
- Composed of proteins.
- Secreted by both Gram-positive and Gram-negative bacteria into the medium.
- Actively secreted by bacteria.
- Heat-labile, destroyed at 60 °C.
- Have specific actions on tissues.
- More potent in small doses.
- Highly antigenic and can be neutralized by antibodies.
- Toxoid forms can be used for vaccines, such as tetanus toxoid.
Exotoxins and Their Mechanisms of Action
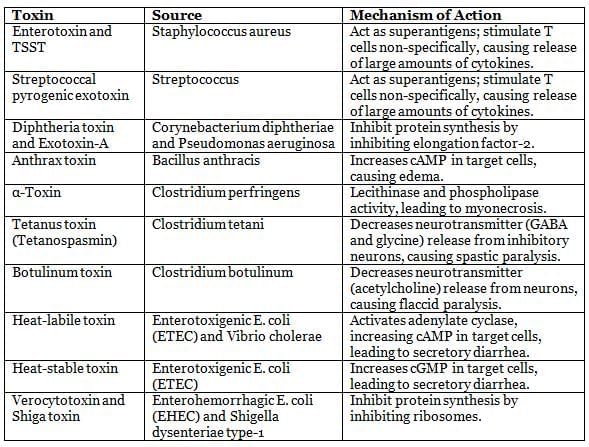
|
75 docs|5 tests
|
FAQs on History, Taxonomy, Morphology and Physiology of Bacteria and Microbial Pathogenicity Chapter Notes - Microbiology - NEET PG
| 1. What is the principle of Transmission Electron Microscopy (TEM) and how does it differ from light microscopy? |  |
| 2. How are bacteria classified in bacterial taxonomy? |  |
| 3. What are the common staining techniques used in microbiology, and what is their purpose? |  |
| 4. What are the key morphological characteristics of bacteria? |  |
| 5. How do bacteria grow and what factors affect their growth and nutrition? |  |




















