Introduction to Acid and Base Chapter Notes | Chemistry for SSS 2 PDF Download
| Table of contents |

|
| Acids and Bases |

|
| Natural Indicators Around Us |

|
| Neutralisation |

|
| Neutralisation in Everyday Life |

|
| Important Points |

|
Acids and Bases
In our daily lives, we encounter many substances with distinct properties, which can be categorized as acidic, basic, or neutral.
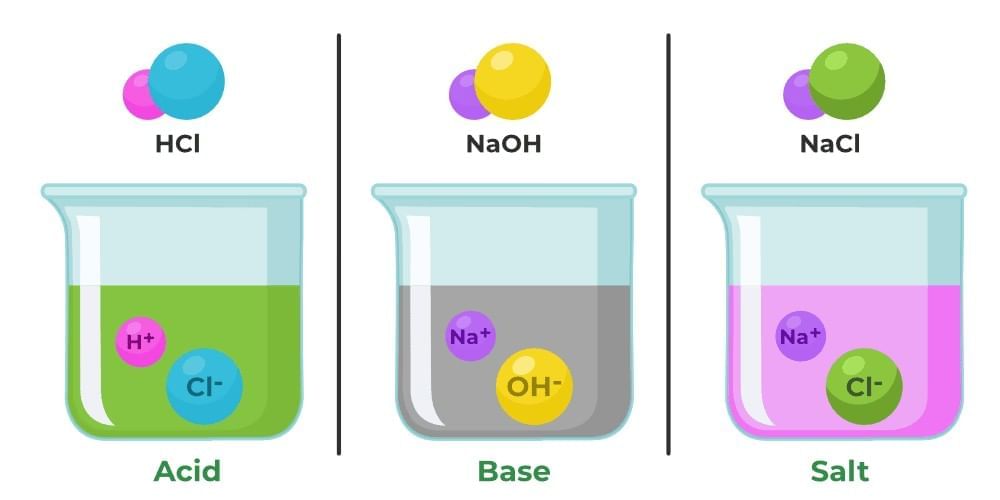
Acids
Acids are substances known for their sour taste (though tasting them is not safe). Examples of acidic substances include curd, vinegar, lemon, and orange juice, which all contain natural acids. The word "acid" is derived from the Latin word 'ACERE', meaning sour.
Properties of Acids:
- Sour in taste.
- Turn blue litmus paper red.
- Turn China rose solution dark pink or magenta.
Bases
Bases are substances with a bitter taste and a slippery feel. Common bases include baking soda, milk of magnesia, and soap. The chemical nature of these substances is referred to as basic.
Properties of Bases:
- Bitter in taste.
- Turn red litmus paper blue.
- Turn turmeric paper reddish-brown.
- Turn China rose solution green.
Indicators
There are special substances that are used to test whether something is acidic or basic. These substances are called indicators. Indicators change their colour when added to an acidic or basic solution. Some natural indicators are litmus, turmeric, and china rose petals.
 Natural Indicators
Natural Indicators
Types of Indicators
Indicators are of two types- natural indicators and synthetic indicators
- Natural Indicators - These indicators are obtained from naturally occurring substances.Example: litmus paper, Chinese rose, turmeric
- Synthetic Indicators - These indicators are made in a laboratory. Example: methyl orange, phenolphthalein.
Natural Indicators Around Us
(i) Litmus: A Natural Dye
Litmus is a common natural indicator extracted from lichens. Litmus has a purple colour in a neutral medium, red colour in an acidic medium, and blue in a basic medium.
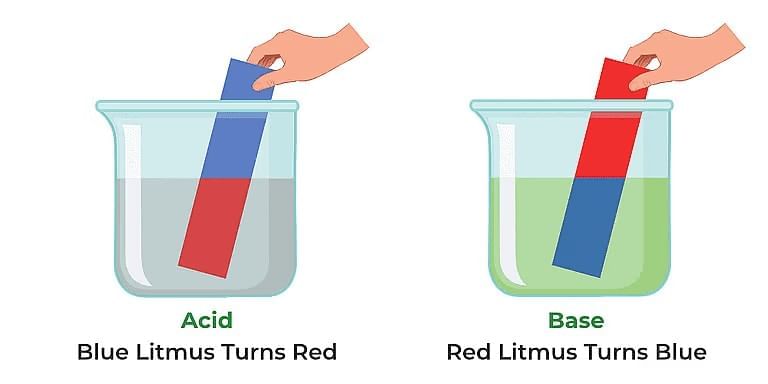 Reaction of litmus paper with acid and base
Reaction of litmus paper with acid and base
- When a drop of freshly prepared lemon juice is put on red litmus, it remains red, indicating the acidic nature of lemon juice.
- If blue litmus is used instead, then it turns red, again indicating the acidic nature of lemon juice.
- A solution of litmus turns red if it is acidic and blue if it is basic.
(ii) Turmeric
Turmeric changes colour to indicate acidic and basic substances.
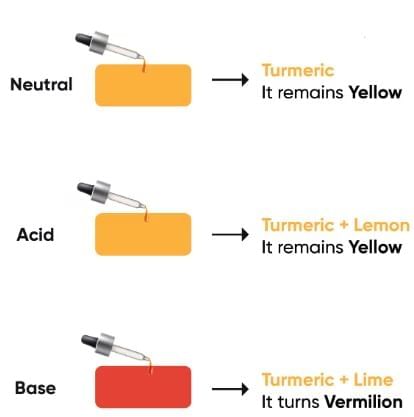 Reaction of turmeric with neutral, acidic and basic solutions
Reaction of turmeric with neutral, acidic and basic solutions
- Turmeric paper changes colour in a basic medium.
- In an acidic medium, it remains yellow, while in a basic medium, it turns reddish-brown.
- When a drop of soap solution is put on turmeric paper, it turns red. This indicates the basic nature of soap solution.
(iii) China Rose
China rose(Gudhal petals) is also a natural indicator extracted from China rose petals. A solution of china rose turns green in a basic solution and bright pink or magenta in an acidic solution.
 Reaction of china rose solution with acid and base
Reaction of china rose solution with acid and base
Summary Tables
1. Name of Acid & Bases and their effect on Litmus and Turmeric Paper
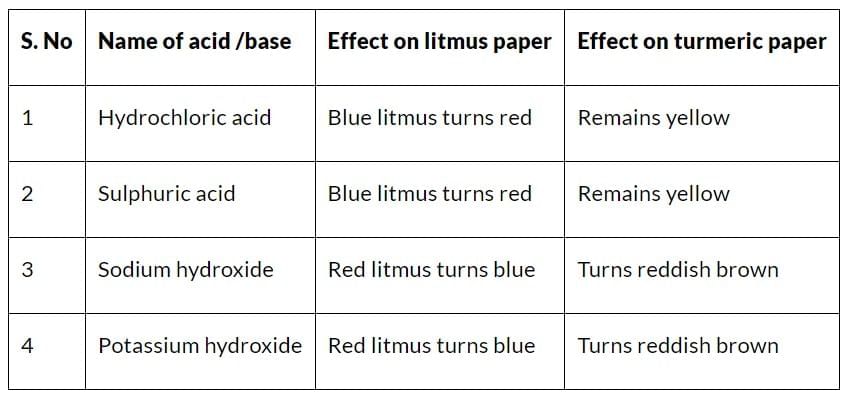
2. Tests Solutions and Effect on Turmeric Paper
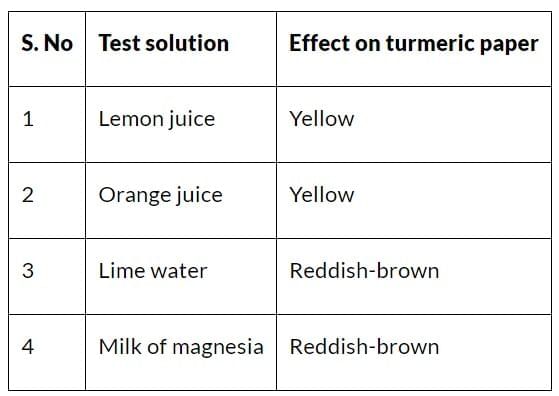
3. Name of Acids and Effect on China Rose
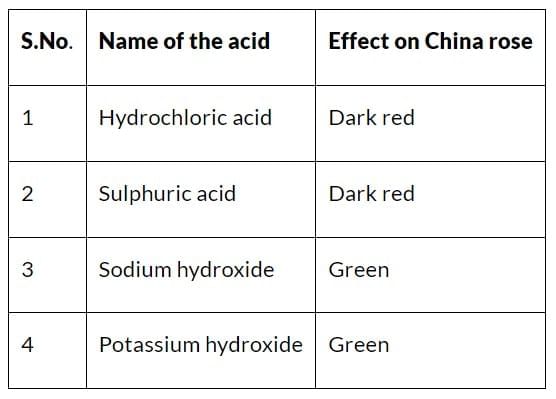
Acid Rain
Acid rain is rain that becomes more acidic than normal due to pollutants in the air. This acid is created when pollutants in the air, such as carbon dioxide, sulphur dioxide and nitrogen dioxide, dissolve in rainwater, and form acids like carbonic acid, sulphuric acid and nitric acid.
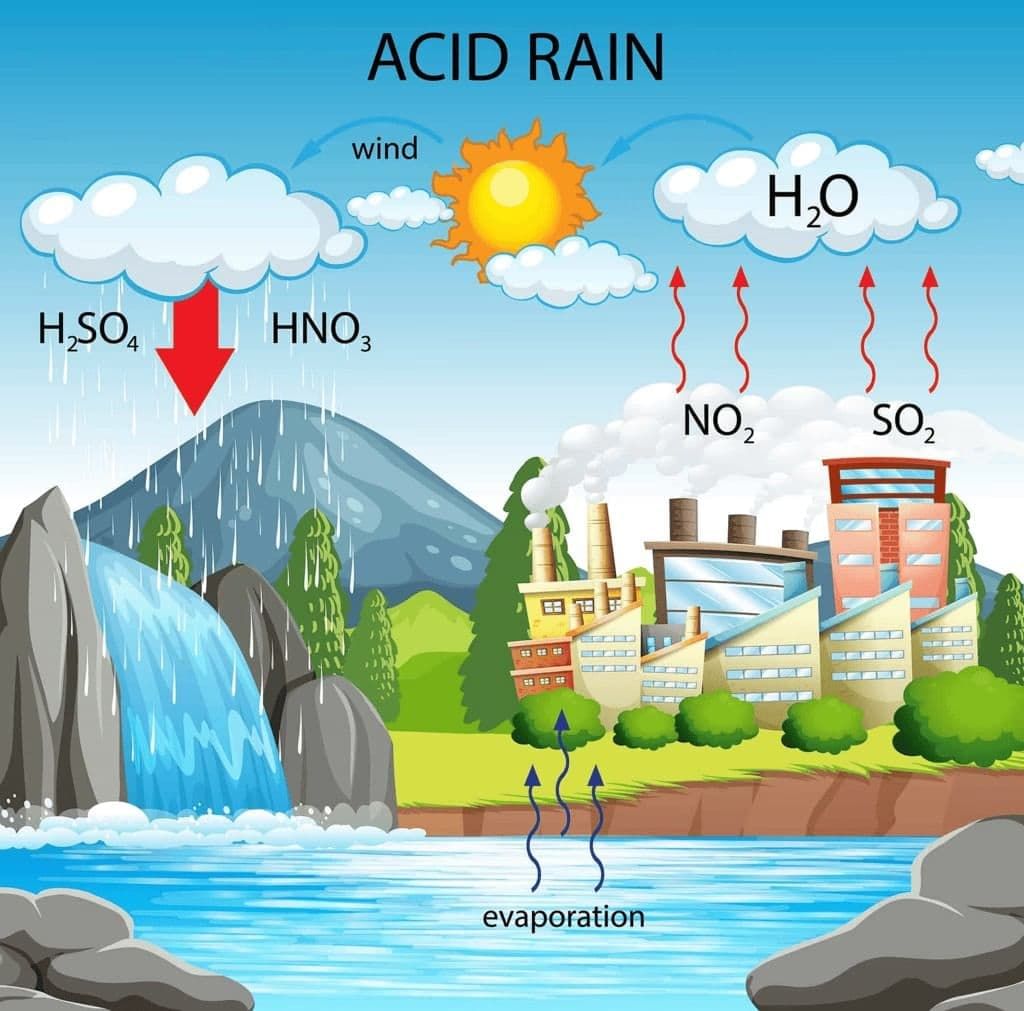 Acid Rain
Acid Rain
- This rain causes a lot of damage to buildings, plants and animals.
- The acidity in the rain can erode structures, damage plant life, and affect aquatic ecosystems.
- This is one important reason why we must avoid polluting our atmosphere.
Neutralisation
Neutralization is a chemical reaction in which an acid reacts with a base to produce salt and water. This reaction also releases heat, making it an exothermic process.
Example of a Neutralization Reaction:
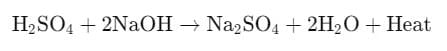
- In this reaction, sulfuric acid (H2SO4) reacts with sodium hydroxide (NaOH) to form sodium sulfate (Na2SO4), water (H2O), and releases heat.
Indicators can be used to identify a neutralization reaction by showing a color change when the reaction occurs. For instance, phenolphthalein is a common indicator used in acid-base reactions.
Example:
- When phenolphthalein is added to sodium hydroxide (NaOH), a base, the solution turns pink.
- As hydrochloric acid (HCl) is added, the pink color gradually fades, eventually turning colorless. This change indicates that neutralization has occurred.
Chemical Reaction:
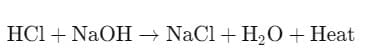 Here, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to produce sodium chloride (NaCl), water, and heat, with the color change in phenolphthalein confirming the completion of the neutralization.
Here, hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to produce sodium chloride (NaCl), water, and heat, with the color change in phenolphthalein confirming the completion of the neutralization.
Neutralisation in Everyday Life
1. Indigestion: The stomach contains hydrochloric acid, which helps in the digestion of food. However, when this acid is produced in excess, it causes indigestion, which is painful. An antacid such as milk of magnesia neutralises the excessive acid in the stomach and provides relief from the pain due to indigestion. Milk of magnesia
Milk of magnesia
2. Ant Bite: Ant bite contains formic acid. When an ant bites, it injects this formic acid into the skin. This causes pain. It can be neutralised by rubbing the ant bite with moist baking soda, which contains sodium hydrogen carbonate (NaHCO3), or with a solution of calamine, which contains zinc carbonate (ZnCO3).
3. Soil Treatment: Excessive use of fertilisers makes the soil acidic. As a result, plants cannot grow properly, and the yield decreases. Adding a base like quick lime(calcium oxide) or slaked lime (calcium hydroxide) neutralises the soil and makes it suitable for plants.
Similarly, when soil is basic in nature, organic matter is added to release acid and make it neutral and, thus, suitable for plants.
4. Factory Waste: Factory waste contains acids. This waste must be treated with bases for neutralization before it is released into a water source. Otherwise, it can damage living organisms in the water source.
Important Points
- Acids have a sour taste.
- Acids turn blue litmus paper red.
- Acids cause China rose solution to turn dark pink.
- Bases have a bitter taste.
- Bases turn red litmus paper blue.
- Bases turn turmeric paper or solution red.
- Bases cause China rose solution to turn green.
- Indicators are special substances that change color to show whether a substance is acidic or basic.
- Natural indicators include litmus, turmeric, and China rose solution.
- Litmus is a natural dye obtained from lichens.
- When an acid and a base are mixed in the correct ratio, they neutralize each other, resulting in the formation of salt and water. This process is known as neutralization or a neutralization reaction.
- The salt formed during a neutralization reaction may be either acidic or basic, depending on the strength of the acid and base involved.
|
1 videos|45 docs|16 tests
|
FAQs on Introduction to Acid and Base Chapter Notes - Chemistry for SSS 2
| 1. What are natural indicators and how do they work to identify acids and bases? |  |
| 2. What is neutralization and what are some examples of it in everyday life? |  |
| 3. How are acids and bases classified and what are their properties? |  |
| 4. What role do neutralization reactions play in environmental processes? |  |
| 5. Why is it important to understand acids and bases in the context of health and safety? |  |














