Introduction to Chemistry Chapter Notes | Chemistry Class 6 ICSE PDF Download
Introduction
Chemistry is a part of science that helps us learn about the things around us, like food, clothes, and medicines. It tells us what things are made of, how they change, and how they work together. Chemistry is very important because it makes our lives better by helping us create useful things like soaps, medicines, and fertilizers. In this chapter, we will learn what chemistry means, how it started, why it is important, and how it is used in food, clothes, cosmetics, and industries.
Chemistry – Meaning
- Science is about studying and learning through experiments and observations.
- Science has two main parts: physical science and natural (life) science.
- Physical science includes Physics, Chemistry, and Biology.
- Chemistry is the study of what things are made of, their properties, and how they change when mixed with other things.
- Chemistry helps us understand the world and answer questions about how things work.
- Everything around us, like the air we breathe, the food we eat, and the clothes we wear, is connected to chemistry.
Development of Chemistry – A Historical Perspective
Alchemy
- Alchemy was an old study that mixed science with spiritual ideas.
- It was used to learn about nature and how things work.
- Alchemy had both scientific and magical parts.
- People who did alchemy were called alchemists.
- Alchemists were some of the first scientists who studied chemistry.
- They worked with metals, inks, paints, cosmetics, and made extracts and liquids like perfumes.
- Alchemists were the first to separate zinc and phosphorus from other materials.
- Their work helped humans move forward in civilization.
- But alchemists also believed in magic and thought their work was like magic spells or rituals.
- They did not follow a proper scientific method like we do today.
The physical aspect of alchemy:
- Alchemy focused on changing simple metals into silver or gold.
- Alchemists tried to find a magical thing called the "philosopher's stone."
- They believed the philosopher's stone could turn metals like iron and copper into gold when heated with them.
- People thought gold was the best and most perfect metal.
- Gold was also believed to make people healthy and live forever.
- Alchemists were not successful in making gold with the philosopher's stone.
- But while trying, they learned a lot about different metals.
- They also found new ways to take out metals from rocks and mix metals to make alloys.
Development of modern chemistry:
- By the late 1700s, chemistry became a separate subject from alchemy.
- Alchemy had a big role in starting modern chemistry.
- Modern chemistry uses experiments and the scientific method to learn new things.
- Alchemists were the first to find new elements and compounds.
- They also used these discoveries to make medicines that helped humans.
Did You Know?
- Nagarjuna (931 CE) was an Indian chemist who wrote a book called Rasaratnakara. The book talked about how to get metals like silver and copper from their ores. It also explained the processes of distillation (turning liquids into gas and back) and sublimation (turning solids into gas).
- The Iron Pillar near Qutub Minar in Delhi is 7 meters tall and does not rust. It is made of wrought iron, which has not rusted even after many years. The pillar has a layer of crystalline iron hydrogen phosphate that stops rusting. This layer protects the Iron Pillar from Delhi’s weather.
Notable Chemists
Chemists are scientists who study and do experiments in chemistry. Let’s learn about some famous chemists and what they did.
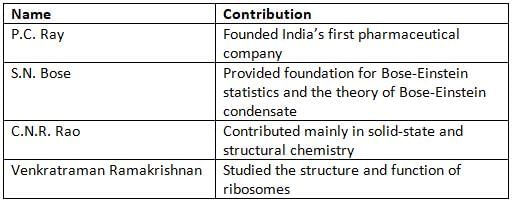
Antoine Lavoisier
- Antoine-Laurent de Lavoisier is called the "Father of Modern Chemistry."
- He discovered the role of oxygen in burning (combustion).
- He gave the "Law of Conservation of Mass," which says that mass does not change in a chemical reaction.
- Lavoisier also named the elements oxygen and hydrogen.
Dmitri Mendeleev
- Dmitri Ivanovich Mendeleev was the first scientist to create the modern periodic table of elements.
- The periodic table arranges elements based on their properties.
- Mendeleev’s table helped predict the existence and properties of new elements.
John Dalton
- John Dalton proposed the modern atomic theory.
- He gave the "Law of Multiple Proportions," which explains how elements combine in fixed ratios.
- He also gave the "Law of Partial Pressures," which explains how gases behave when mixed.
Some More Scienties
J.J. Thomson
- J.J. Thomson discovered the electron, a tiny particle in atoms.
- He proposed the "Plum Pudding Model" of an atom, saying electrons are inside a cloud of positive charge.
- His model explained how positive and negative charges balance in an atom.
- Thomson’s work helped us understand the structure of atoms better.
Ernest Rutherford
- Ernest Rutherford did the famous "gold foil experiment."
- He found that an atom has a small, dense nucleus in the center, surrounded by electrons.
- Rutherford also discovered the concept of radioactive half-life (how long it takes for half of a radioactive substance to decay).
Niels Bohr
- Niels Henrik David Bohr developed the "Bohr model" of an atom, also called the Bohr atomic model.
- He said that electrons move in fixed energy levels (orbits) around the nucleus of an atom.
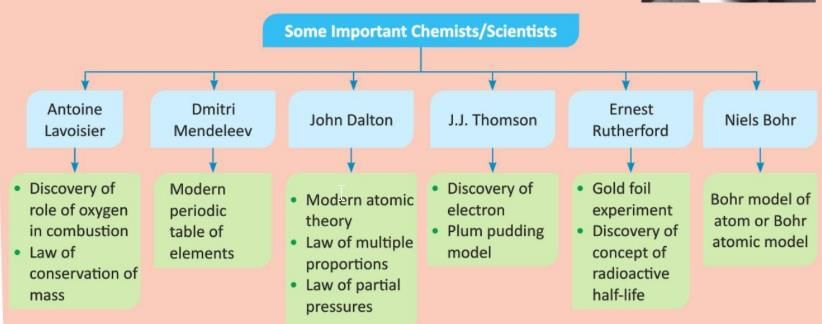
Some Other Notable Scientists and their Discoveries
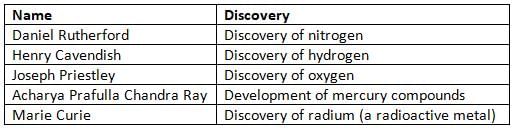
Notable Indian Chemists/Scientists and their Contribution
Importance of Chemistry
- Chemistry is very important in our daily lives and plays a big role in many areas.
- We use chemistry directly or indirectly in things like food, clothes, shelter, medicines, hygiene, fertilizers, drugs, and entertainment.
- Chemical processes help make many useful things that we need every day.
- Chemistry helps us understand how things in our environment work.
Food and Chemistry
As the population grows, we need more food, so preserving food is important to avoid wastage. Chemistry helps preserve food and make it last longer for people to use.
Food and Its Preservatives
- Preservatives are chemicals that stop food from spoiling and make it last longer.
- Sodium benzoate is used to preserve food items like fruit products, drinks, and salads.
- Sugar, salt, and vinegar are used as preservatives to stop bacteria from growing in food.
- Sorbic acid and its sodium and potassium salts are used to preserve dairy products, fish, seafood, fruits, vegetables, baked foods, and cookies.
- Other preservatives include sodium metabisulphite, salicylic acid, benzoic acid, and propionic acid.
Fun Fact
New methods in farming have increased crop production and helped grow more food.
- Fertilizers: These are chemicals that give crops the nutrients they need to grow better, like urea, sodium nitrate, potassium, and ammonium phosphate.
- Pesticides: Farmers use these to kill pests that harm crops, such as aldrin, malathion, and parathion.
- Insecticides: These are chemicals that kill insects affecting crops, like benzene hexachloride (BHC), dichlorodiphenyltrichloroethane (DDT), and carbaryl.
- Fungicides: These are chemicals that protect crops from fungi, like sulphur, calcium polysulphide, and ethylene oxide.
Food Processing
- Food processing means changing raw materials into food products using physical or chemical methods.
- Processed foods are made for eating, cooking, or storing and have a longer shelf life.
- Various vitamins and minerals are added to processed foods to make them more nutritious.
- Examples of processed foods are cheese, bread, jam, jelly, butter, snacks, soft drinks, tinned food, meat products, yogurt, and ice cream.
Cosmetics and Chemistry
- Cosmetics are products used to clean, beautify, or improve the look of our body.
- Examples of cosmetics include skin creams, lotions, moisturizers, lipsticks, talcum powder, foundation, shampoo, hair styling products, gel, hair spray, deodorants, and perfumes.
- We use cosmetics every day to stay clean and look good.
- Chemistry plays a big role in making cosmetics by mixing different chemical ingredients.
- For example, lipsticks are made from oils, waxes, pigments, and skin softeners called emollients.
- Talcum powder is made from talc, a mineral that contains magnesium, silicon, oxygen, and hydrogen.
Clothing and Chemistry
- Chemistry is used in the textile industry to make clothes from fibers.
- Fibers can be natural (like cotton, jute, wool, and silk) or synthetic (man-made).
- In the past, natural fibers like cotton, jute, wool, and silk were used to make dresses, sarees, bags, shawls, pullovers, and other items.
- Today, synthetic fibers like nylon and terylene are used because they are strong, wrinkle-resistant, and dry quickly.
- Synthetic fibers are used to make dress materials, bed sheets, carpets, bags, blankets, towels, curtains, and many other things.
Medicines and Chemistry
- Chemistry helps us make many medicines to keep us healthy.
- Medicines like antibiotics, such as penicillin and ampicillin, help us fight infections.
- Medicines like aspirin and paracetamol help us feel better when we have fever, pain, or headaches.
- Chemistry studies how different substances work in our body to make these medicines.
- Because of chemistry, the medicine industry is growing bigger and stronger.
- Some common medicines are:
- Aspirin: It helps reduce fever, pain, cold, muscle aches, and headaches.
- Paracetamol: It helps reduce mild to medium pain and fever.
- Penicillin: It is an antibiotic that fights infections caused by bacteria.
Chemicals in Industries
- Chemistry is very important in many industries that make things we use every day.
- It helps make materials like plastics, alloys, synthetic rubber, synthetic fibers, and textiles.
- Chemicals are used to create different products in industries, which are important for our lives.
- Industries use chemicals to make:
- Paints, textiles, stain removers, soaps, and detergents.
- Fertilizers, drugs, pesticides, glass, and synthetic fibers.
- Chemical processes also help in:
- Making common salt from sea water.
- Getting sugar from sugarcane juice.
- Taking out metals from their ores.
- Making alloys and developing medicines.
- Chemical industries are very important because they make many products we need.
- Chemistry helps improve our lives by making new and better things for us to use.
- It also helps the world economy grow and makes life better for people.
Some More Benefits of Chemistry
- Petrochemicals: Petrochemicals are products made from petroleum. They include petrol, diesel, kerosene, LPG, paints, varnishes, wax, and paraffin.
- Building materials: Chemical processes help make materials like cement, mortar, steel, and bricks for building.
- Transport: Chemistry helps make fuels like petrol and diesel for buses, scooters, cars, trains, ships, and aeroplanes.
- Electronics: Chemistry helps make semiconductors like silicon and germanium. These are used in electric circuits for radios, computers, calculators, and solar cells.
- Extraction of metals: Metals are taken out from their ores using chemical processes. These metals are used to make things for industries and homes.
- Space research: Chemistry helps make better and stronger fuels for space technology. It helps us launch space satellites successfully.
Fun Fact
Chemistry has done a lot of good, but it also has some bad effects.
- Pollution:
- Smoke from vehicles and factories causes air pollution.
- Insecticides and pesticides pollute soil and water.
- Industrial waste and sewage also pollute water.
- Combustion of petrol and diesel makes harmful gases.
- These gases damage the ozone layer and cause global warming.
- Destructive chemicals and weapons:
- Some chemicals like RDX, TNT, gunpowder, and atom bombs are very harmful.
- They can cause a lot of destruction and kill many people.
- Nuclear reactors used for power also create nuclear waste, which is a big problem.
- Side-effects of drugs:
- Some drugs can disturb our body’s hormones.
- They can cause problems like anxiety, insomnia, or other health issues.
- Depletion of natural resources:
- Too much use of chemicals can harm nature.
- Industries use up resources like coal, petroleum, minerals, and forests.
- Deforestation increases carbon dioxide in the air, causing global warming.
Point To Remember
- Science is the study of knowledge and ongoing processes in the world.
- Chemistry is a part of science that studies substances, their properties, and how they react with each other.
- Chemistry is very important and plays a big role in all parts of life.
- Alchemy was an old study that mixed chemistry with spiritual ideas and metalwork.
- The physical part of alchemy was about changing base metals into silver or gold.
- Famous scientists like Antoine Lavoisier, Dmitri Mendeleev, John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr made big contributions to chemistry.
- Chemistry helps in many areas of life, like food, farming, medicine, healthcare, cosmetics, clothing, and more.
Glossary
- Science: It is the study of nature and its processes using experiments and observations.
- Chemistry: It is a part of science that studies substances, their properties, and how they mix with each other.
- Alchemy: It is an old study that mixed chemistry with spiritual ideas and metalwork.
- Alchemists: People who studied alchemy.
- Preservatives: Chemicals used to keep food fresh and stop it from getting spoiled.
- Food processing: Changing raw food into ready-to-eat products using physical or chemical methods.
- Cosmetics: Products used to make the human body look better on the outside.
|
8 videos|40 docs|5 tests
|
FAQs on Introduction to Chemistry Chapter Notes - Chemistry Class 6 ICSE
| 1. What is the significance of chemistry in our daily lives? |  |
| 2. Who are some notable chemists in the history of chemistry? |  |
| 3. How does chemistry affect the food we eat? |  |
| 4. In what ways is chemistry involved in the production of medicines? |  |
| 5. How is chemistry integrated into cosmetics and personal care products? |  |
















