Language of Chemistry Chapter Notes | Chemistry Class 9 ICSE PDF Download
Introduction
Chemistry is the study of matter, including its composition, properties, and the changes it undergoes. The "Language of Chemistry" chapter introduces the basic terms and concepts used to describe elements, compounds, and chemical reactions. It covers symbols for elements, valency, chemical formulae, and equations, helping us understand how matter is represented and how reactions are written and balanced. This chapter also explains how to calculate the relative atomic and molecular masses and the percentage composition of compounds, providing a foundation for further studies in chemistry.
- Chemistry studies matter, its nature, structure, and changes under different conditions.
- Matter is a complex relationship between elements, which are pure substances that cannot be broken down into simpler substances.
- Elements are made of atoms, the smallest particles that show all properties of an element.
- A group of atoms of the same element forms a molecule, which can exist independently and shows the element's properties.
- Molecules can be:
- Monoatomic: One atom (e.g., helium, neon).
- Diatomic: Two atoms (e.g., hydrogen - H₂, oxygen - O₂, nitrogen - N₂).
- Tetratomic: Four atoms (e.g., phosphorus - P₄).
- Octatomic: Eight atoms (e.g., sulphur - S₈).
- Compounds are formed when atoms or molecules of different elements combine (e.g., sodium chloride - NaCl, water - H₂O, ammonia - NH₃).
- Before 1600 A.D., alchemists used pictographic symbols (e.g., triangle for earth, crescent for silver).
- Dalton used symbols like a circle for oxygen and a circle with a dot for hydrogen.
- Johann Berzelius suggested using the first letter of an element’s name (in English or Latin) as its symbol, forming the basis of the IUPAC system.
Symbols of certain elements based on their Latin names
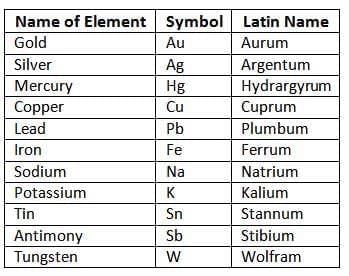
However, the approach proposed by him became the foundation for the IUPAC (International Union of Pure and Applied Chemistry) system used for chemical symbols and formulas.
Chemical Symbols
- A symbol is a short way to represent an element’s atom or its name.
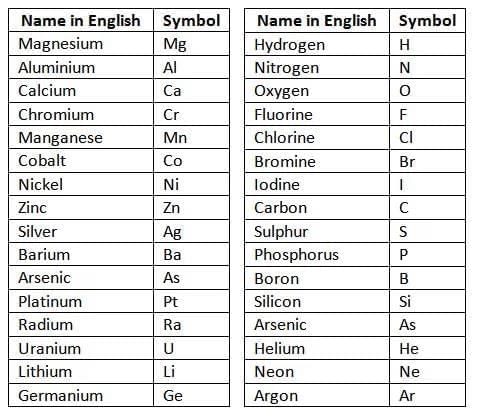
- Usually, the first letter of the element’s name (in English or Latin) is used, written in capital (e.g., S for sulphur, H for hydrogen).
- If multiple elements start with the same letter, two letters are used: the first in capital, the second in lowercase (e.g., C for carbon, Co for cobalt, Cu for copper from Latin 'cuprum').
- Significance of a symbol:
(i) Name of the element
Table of symbols for common elements:
(ii) One atom of the element
(iii) Quantity of the element equal in mass to its atomic mass or gram atomic mass.
For example, the symbol N stands for
(i) The element Nitrogen
(ii) One atom of Nitrogen
(iii) 14 parts by weight of Nitrogen. This weight being the atomc weight of the element.
Note: Symbols are case-sensitive (e.g., Co is cobalt, CO is carbon monoxide, a compound).
Formula
- A chemical formula shows the molecule of an element or compound using symbols of its atoms.
- Element molecules may have one or more atoms (e.g., H₂, O₂, N₂, Cl₂, Br₂, I₂ for diatomic elements).
- Compound formulas show the number of each atom in a molecule using subscripts (e.g., NH₄Cl has 1 nitrogen, 4 hydrogens, 1 chlorine; Na₂CO₃ has 2 sodiums, 1 carbon, 3 oxygens).
- Significance of a molecular formula:
- Shows the molecule and its molecular mass.
- Indicates the number of different atoms in one molecule.
- Shows the mass ratio of elements in the compound.
- Example: CO₂ means:
- Molecular formula is CO₂.
- One carbon atom is bonded to two oxygen atoms.
- Molecular mass is 44 amu (C = 12, O = 16 × 2 = 32).
Valency
- Valency is the combining capacity of an atom or radical, measured by the number of hydrogen or double the number of oxygen atoms it can combine with.
Modern definition
Valency is the number of electrons an atom can lose, gain, or share in a chemical reaction.
Elements that have one, two, or three electrons in their outermost shell (valence shell) are generally called metals. The electrons present in this
outer shell are referred to as valence electrons. To achieve a stable electronic arrangement, these atoms give up their valence electrons and
become positive ions.

- Non-metals (5–7 electrons in outer shell) gain electrons to form negative ions (anions):
- Note: Elements with 4 electrons Carbon (non-metal), Silicon, Germanium (metalloids), others (metals).

- Valency based on electron count in outer shell:

- Examples of valency by sharing:
- One atom of chlorine combines with one hydrogen atom to form a molecule of hydrogen chloride. So, the valency of chlorine is one.
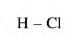
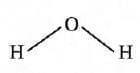
- One atom ot oxygen combines with 2 atoms or hydrogen to form a molecule of water. So, the valency of oxygen is two.
- In an ammonia molecule, one atom of nitrogen combines with 3 atoms of hydrogen. So, the valency of nitrogen is three.
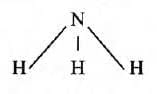
- In a methane molecule, one carbon atom combines with 4 hydrogen atoms. So, the valency of carbon is four.
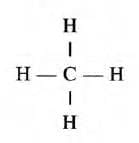
Variable valency
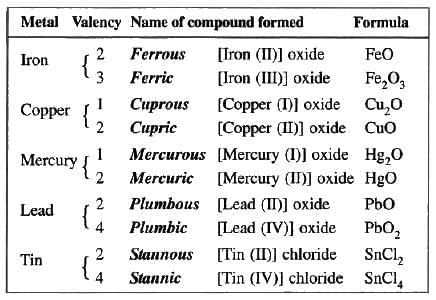
Certain elements exhibit more than one valency, i.e. they show variable valency.Reasons for variable valency
An atom of an element can sometimes lose more electrons than are present in its valence shell, i.e. there is a loss of electrons from the penultimate shell too. Therefore, such an element is said to exhibit variable valency.
If an element exhibits two different positive valencies, then we use the suffix “ous” for the lower valency and the suffix “ic” for the higher valency. Modem chemists use Roman numerals in place of these trivial names. For example, SnCl2, i.e. stannous chloride is written as Tin (II) chloride, SnCl4, i.e. stannic chloride is written as Tin (IV) chloride.
Non-metals like nitrogen, phosphorus and sulphur also show variable valency. Nitrogen and phosphorus exhibit valencies of 3 and 5 while sulphur exhibits valencies of 2, 4 and 6.
Examples of variable valency
Radicals
The molecule of a compound generally consists of two parts, and these individual parts are called radicals. For instance, in a potassium chloride molecule, there are two parts—potassium and chloride. Hence, potassium is one radical, and chloride is the other. In the same way, magnesium sulphate contains magnesium and sulphate as its radicals.
For instance, in a potassium chloride molecule, there are two parts—potassium and chloride. Hence, potassium is one radical, and chloride is the other. In the same way, magnesium sulphate contains magnesium and sulphate as its radicals.
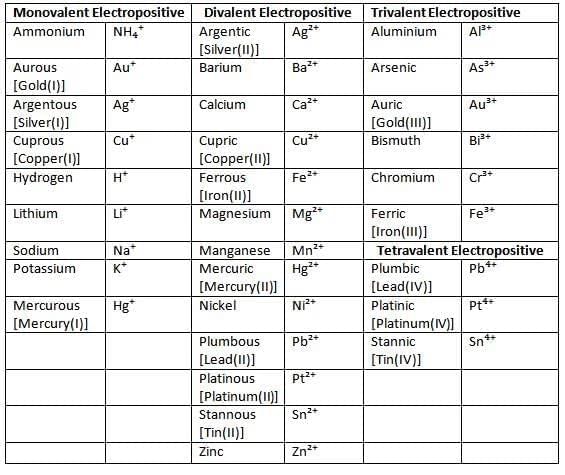
- A radical is an atom or group of atoms acting as a single unit with a positive or negative charge.
- Basic radicals (positive, cations): e.g., NH₄⁺ (ammonium), Na⁺ (sodium).
- Acidic radicals (negative, anions): e.g., Cl⁻ (chloride), CO₃²⁻ (carbonate).
- Example: In MgSO₄, Mg²⁺ is electropositive (basic), SO₄²⁻ is electronegative (acidic).
List of some common electrovalent positive ions (basic radicals)
List of some common electrovalent negative ions (acid radicals)
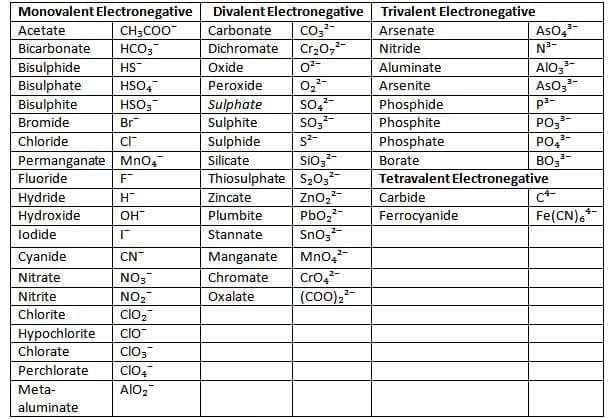
Writing Chemical Formulae
- Radicals have their own combining power (valency) to form chemical formulae.
- Criss-cross method to write formulae:
- Write symbols: Basic radical first, then acidic radical.
- Write valency on top of each symbol.
- Divide valencies by their highest common factor (HCF) to get the simplest ratio.
- Interchange valencies and write as subscripts. Enclose radical groups in brackets if valency > 1.
- Example table for writing formulae:
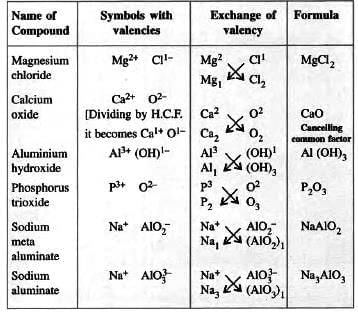
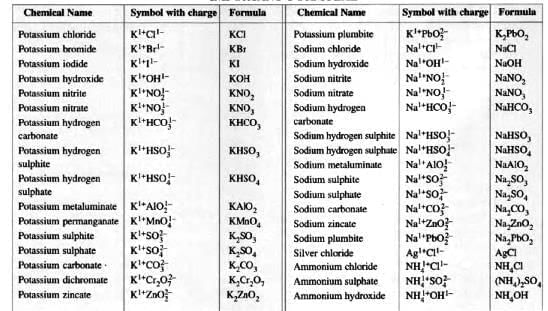
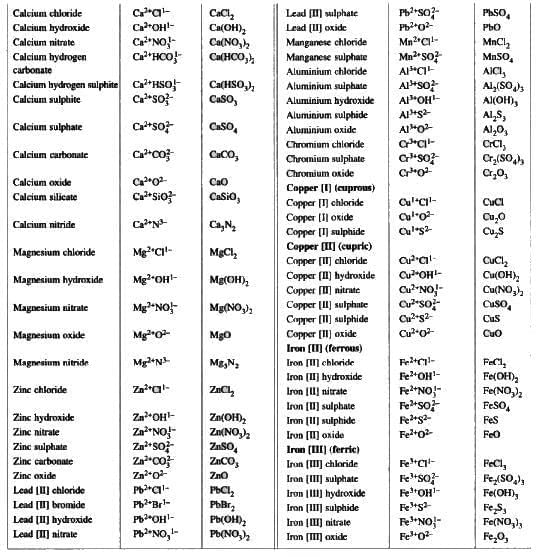
Naming Certain Compounds
- Metal and non-metal: Metal first, non-metal with suffix -ide (e.g., Ca₃N₂ - calcium nitride).
- Two non-metals: Use prefixes like tri-, tetra-, penta- (e.g., PCl₃ - phosphorus trichloride, PCl₅ - phosphorus pentachloride).
- Two elements and oxygen:
- Less than 2 oxygen atoms: Prefix 'hypo' (e.g., NaClO - sodium hypochlorite).
- 2 oxygen atoms: Suffix 'ite' (e.g., NaClO₂ - sodium chlorite).
- 3 oxygen atoms: Suffix 'ate' (e.g., NaClO₃ - sodium chlorate).
- More than 3 oxygen atoms: Prefix 'per' (e.g., NaClO₄ - sodium perchlorate).
- Naming acids:
- Binary acids: Prefix 'hydro' and suffix 'ic' (e.g., HCl - hydrochloric acid, HF - hydrofluoric acid).
- Acids with polyatomic groups: Named after the second element, no 'hydro' (e.g., H₂SO₄ - sulphuric acid, HNO₃ - nitric acid, H₃PO₄ - phosphoric acid).
- Fewer oxygen atoms: Suffix 'ous' (e.g., H₂SO₃ - sulphurous acid, HNO₂ - nitrous acid).
- Trivial names: Common names not following systematic rules (e.g., NH₃ - ammonia, H₂O - water).
To Calculate the Valency from the Formula
The valency of elements can be determined based on the knowledge of the valencies of negative radicals and of the fact that the valencv of:


Chemical Equation
- A chemical equation uses symbols and formulas to show a chemical reaction.
- For instance, when coal burns in air, it reacts to form carbon dioxide, which is a chemical reaction.
- This reaction can be expressed in two ways: as a word equation or a chemical equation (using symbols and formulas), as shown below:

Steps to write a chemical equation:
- On the left side, write the symbols or formulas of the reactants, separated by a (+) sign.
- On the right side, write the symbols or formulas of the products, also separated by a (+) sign.
- Place an arrow (→) between the reactants and products to show the reaction direction.
- Use the molecular forms of reactants and products since their atomic forms are typically not stable or able to exist independently.
- Example: Sodium reacts with water to produce sodium hydroxide and hydrogen gas.
2Na + 2H₂O → 2NaOH + H₂
- A chemical equation indicates the substances that react (called reactants) and the substances that are produced (called products).
- Example: Sodium reacts with water to produce sodium hydroxide and hydrogen gas.
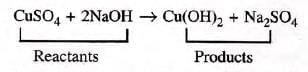
- Example:. Here, copper sulphate and sodium hydroxide are the reactants, while copper hydroxide and sodium sulphate are the products.
Chemical reactions may involve:
- One reactant producing two or more products.
- Two reactants forming one product.
- Two reactants forming two products.
- Two reactants forming three or more products.
(i) One reactant and two or more products:
- Examples:
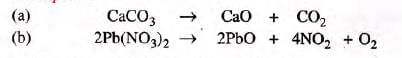
(ii) Two reactants and one product:
- Examples:
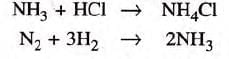
(iii) Two reactants and two products:
- Examples:
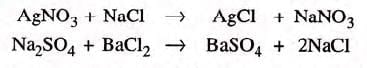
(iv) Two reactants and three or more products:
- Examples:

Skeleton equation: This is an unbalanced chemical equation where the number of atoms of each element on both sides is not equal.
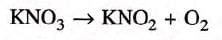
- In this equation, the number of oxygen atoms on the left side (in KNO₃) does not match the number of oxygen atoms on the right side (in KNO₂ and O₂), making it unbalanced.
Balanced Equation
A balanced equation has an equal number of atoms of each element on the reactant side (left) and the product side (right).
Examples:
- (a) CaCO₃ → CaO + CO₂
- In this equation, the number of calcium (Ca), carbon (C), and oxygen (O) atoms is the same on both sides, meaning the equation is balanced.
- (b) KNO₃ → KNO₂ + O₂
- In this equation, the number of oxygen atoms is not the same on both sides, so it is unbalanced.
- The balanced form of this equation is:
2KNO₃ → 2KNO₂ + O₂
How to balance a chemical equation
There are two methods of balancing an equation: (i) Hit and trial method (ii) Partial equation method
1. Balancing by hit and trial method
This method consists of counting the number of atoms of each element on both sides and trying to equalize them. Take the following steps: (i) Count the number of times (frequency) an element occurs on either side. (ii) The element with the least frequency is balanced first.
Example 1: Balance the following equation

Solution :
Step 1: Count the number of atoms of all the elements on either side of the chemical equation.
Element Reactant side Product side
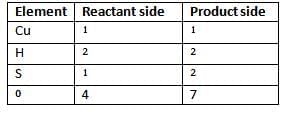
Step 2: Copper and hydrogen are equal on both sides so to equalise sulphur atoms multiply H2S04 by 2.

Step 3: To equalise hydrogen atoms, multiply H20 by 2.
This gives the balanced equation.

Example 2 : Balance the following skeleton equation.
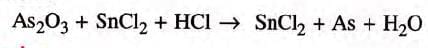
Step 1: Count the number of atoms of all the elements on both sides of the equation.
Element Reactant side Product side
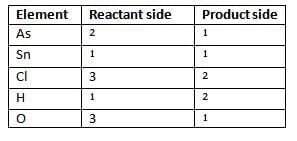
Step 2: To equalise As and O, multiply As by 2 and H20 by 3.
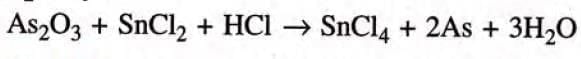
Step 3: To equalise H atoms multiply HC1 by 6.
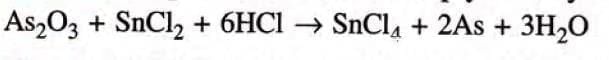
Step 4: Multiply SnCl2 and SnCl4 both by 3.
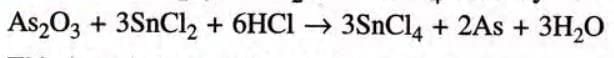
This is te balanced chemical equation.
Information Conveyed by a Balanced Chemical Equation
Refer to the following equations :
2NaOH + H₂SO₄ → Na₂SO₄ + 2H₂O
The equation provides the following details:
- The outcome of the chemical reaction.
- The reactants that participate and the products that are formed as a result of the reaction.
- The number of molecules of each substance involved in the reaction. For example, two molecules of sodium hydroxide react with one molecule of sulphuric acid to produce one molecule of sodium sulphate and two molecules of water.
- The composition of each molecule. For instance, one molecule of sodium hydroxide (NaOH) consists of one sodium atom, one oxygen atom, and one hydrogen atom.
- The molecular mass of the substances: 80 parts by weight of sodium hydroxide reacts with 98 parts by weight of sulphuric acid to form 142 parts by weight of sodium sulphate and 36 parts by weight of water
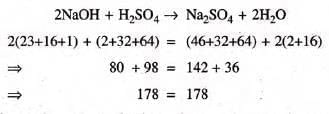
- According to the given equation, 178 grams of reactants yield 178 grams of products.

- The balanced equation provides the following details:
- (a) One molecule of calcium carbonate reacts with two molecules of hydrochloric acid to form one molecule each of calcium chloride, water, and carbon dioxide.
- (b) 100 grams of calcium carbonate reacts with 73 grams of hydrochloric acid to produce 111 grams of calcium chloride, 18 grams of water, and 44 grams of carbon dioxide.
- (Note: All masses are measured in grams.)
- (c) 100 grams of calcium carbonate, when reacted with 73 grams of HCl, will produce 22.4 liters of carbon dioxide at standard temperature and pressure (STP).
Limitations of a Chemical Equation
Does not show:
- Physical state (solid, liquid, gas).
- Time taken for reaction.
- Heat absorbed or released.
- Concentrations of reactants/products.
- Reaction rate.
- Reversible or irreversible nature.
- Whether reaction completes.
A chemical equation can be made more informative by writing the following additional information to it.
- Add temperature, pressure, catalyst above/below arrow (e.g., N₂ + 3H₂ → 2NH₃ [Fe+Mo, 450°C, 200-900 atm]).
- Show physical states: (s) for solid, (l) for liquid, (g) for gas, (aq) for aqueous (e.g., Zn(s) + HCl(aq) → ZnCl₂(aq) + H₂(g)).
- Indicate heat: +Heat (released), -Heat (absorbed) (e.g., C(s) + O₂(g) → CO₂(g) + Heat).
- Show concentration (e.g., Mg(s) + H₂SO₄(dil) → MgSO₄(aq) + H₂(g)).
Relative Atomic Mass (Atomic Weight)
- Atoms are too small to weigh directly (e.g., H = 1.66 × 10⁻²⁴ g, C = 1.9926 × 10⁻²³ g).
- Relative atomic mass (RAM) is how many times an atom is heavier than 1/12th of a C-12 atom.
- Atomic mass unit (amu or u) = 1/12th mass of C-12 (1.6605 × 10⁻²⁴ g).
- Examples: H = 1 amu, O = 16 amu, He = 4 amu.
Relative Molecular Mass (Molecular Weight)
- Relative molecular mass (RMM) is how many times a molecule is heavier than 1/12th of a C-12 atom.
- Calculated by adding atomic masses of all atoms in a molecule.
- Example: H₂SO₄ = (2 × 1) + 32 + (4 × 16) = 98 amu.
- Example calculations:
- CuSO₄·5H₂O: 63.5 + 32 + (16 × 4) + 5(2 + 16) = 249.5 amu.
- (NH₄)₂SO₄: (14 × 2) + (1 × 8) + 32 + (16 × 4) = 132 amu.
- C₁₂H₂₂O₁₁: (12 × 12) + (1 × 22) + (16 × 11) = 342 amu.
Percentage Composition
Percentage composition is the percentage by weight of each element in a compound.
- Formula: % = (Total weight of element / Molecular weight) × 100.
- Example: H₂O (H = 1, O = 16)
- RMM = (2 × 1) + 16 = 18.
- % H = (2 / 18) × 100 = 11.11%.
- Example: Urea (NH₂CONH₂, N = 14, C = 12, O = 16, H = 1)
- RMM = (14 × 2) + 12 + (1 × 4) + 16 = 60.
- % N = (28 / 60) × 100 = 46.67%.
- Example: Na₂CO₃ (Na = 23, C = 12, O = 16)
- RMM = (23 × 2) + 12 + (16 × 3) = 106.
- % Na = (46 / 106) × 100 = 43.4%.
- % C = (12 / 106) × 100 = 11.3%.
- % O = (48 / 106) × 100 = 45.3%.
- Example: Na₂CO₃·10H₂O
- RMM = (23 × 2) + 12 + (16 × 3) + 10(2 + 16) = 286.
- % H₂O = (180 / 286) × 100 = 62.9%.
Empirical Formula of a Compound
- Empirical formula shows the simplest whole-number ratio of atoms in a molecule.
- Example: H₂O₂ → HO (1:1 ratio); C₆H₁₂O₆ → CH₂O (1:2:1 ratio).
- Empirical formula mass = sum of atomic masses in the empirical formula (e.g., HO = 1 + 16 = 17).
Points to remember
- Symbol: Short form for an element’s atom.
- Valency: Combining capacity of an atom/radical, based on electrons lost, gained, or shared.
- Radical: Atom or group acting as a unit with a charge (cation: positive, anion: negative).
- Molecular formula: Shows symbols and number of atoms in a molecule.
- Atomic mass unit (amu): 1/12th mass of C-12 atom.
- Relative molecular mass: Sum of atomic masses in a molecule, compared to 1/12th of C-12.
- Chemical equation: Represents a reaction with symbols/formulae, must be balanced to follow conservation of mass.
- Empirical formula: Simplest ratio of atoms in a molecule.
|
10 videos|47 docs|9 tests
|
FAQs on Language of Chemistry Chapter Notes - Chemistry Class 9 ICSE
| 1. What are chemical symbols and how are they used in chemistry? |  |
| 2. How do you determine the valency of an element? |  |
| 3. What are radicals in chemistry? |  |
| 4. How do you write chemical formulae for compounds? |  |
| 5. What is a balanced chemical equation and why is it important? |  |





















