Materials and Their Structure Chapter Notes | Year 8 Science IGCSE (Cambridge) PDF Download
| Table of contents |

|
| Atoms: The Building Blocks of Matter |

|
| Purity |

|
| Weather and Climate |

|
| Climate and Ice Ages |

|
| The Atmosphere and Climate |

|
| Introduction |

|
| Impact of COVID-19 on Housing Demand and Prices |

|
Atoms: The Building Blocks of Matter
 Atomic Collaboration
Atomic Collaboration
Atoms are extremely small particles that make up everything in the universe. They are so tiny that we need special instruments called microscopes to see them. The term atom comes from a Greek word that means cannot be split, highlighting the fact that atoms are the basic units of matter and cannot be divided into smaller parts without altering their properties. All atoms of a specific element are identical, meaning they have the same characteristics. However, different elements have different types of atoms with their own unique features.
The Basic Structure of an Atom
- Protons and neutrons are located close together in the centre of the atom, known as the nucleus. This is where most of the atom's mass is found because protons and neutrons are much heavier than electrons.
- Electrons move around the nucleus in what is called the electron cloud. They have a negative charge, while protons have a positive charge, and neutrons have no charge.
- The positive charge of protons and the negative charge of electrons create an attraction that holds the atom together.
The Space Inside an Atom
- Between the particles in an atom, there is a lot of empty space. Despite this emptiness, the forces between the particles keep them in place and give the atom its structure.
Development of the Atom Model
- The current understanding of atoms has developed over time through the work of many scientists. In the late 1800s, British scientist J.J. Thompson discovered the electron and proposed a model where electrons were scattered throughout a “soup” of positive charge, known as the plum pudding model.
- Ernest Rutherford, one of Thompson's students, improved this model through the famous gold foil experiment, which showed that atoms are mostly empty space with a dense nucleus.
- In 1932, James Chadwick confirmed the existence of neutrons, refining the atom model we use today.
The Role of Collaboration and Peer Review
- Scientific discoveries often involve collaboration among scientists worldwide. They work together, share ideas, and build on each other's findings, which is essential for advancing our understanding of complex topics like atomic structure.
- After a discovery, other scientists review the work to ensure it is accurate and can be repeated, a process known as peer review.
Ongoing Research on Atomic Structure
- Despite our knowledge about atoms, there is still much to discover. Scientists continue to research atomic structure, such as through the Large Hadron Collider in Switzerland, which conducts experiments to explore the fundamental particles that make up matter and how they interact.
Purity
Pure Elements
- Pure elements consist of only one type of atom. For example, pure gold is made entirely of gold atoms, while gold alloys include other metals like copper or silver.
- When purchasing gold, it is often marked to indicate its purity, such as 24 carat (pure gold), 18 carat, or 9 carat.
- The higher the carat, the more gold the item contains. For example, 18 carat gold has 18 parts gold out of 24, with the remaining 6 parts being other metals, giving it a purity of 75%.
- To calculate the purity percentage, use the formula: ( Parts of gold / Total parts ) × 100.
Understanding Salts
- Salts are compounds formed from acids, and their names indicate which acid was used. For example: Sodium chloride comes from hydrochloric acid. Sodium sulfate comes from sulfuric acid.
Composition of Seawater Salts
- In seawater, the composition of salts is roughly as follows: Sodium chloride : 68% Calcium chloride : 3.1% Magnesium chloride : 14.6% Sodium sulfate : 11.4% Other salts : 2.9%
Purity of Sodium Chloride
- The sodium chloride obtained from seawater is only 68% pure. To calculate the amount of sodium chloride in 1000g of seawater, use: 350g of salts in 1000g of seawater × 68% = 238g of sodium chloride.
Pure Products
- When a chemical reaction produces a product from reactants, it is crucial that the product is pure, especially in medicine. If impurities are present, they could affect the medicine's efficacy.
- In a simple reaction, there is only one product formed. For example: magnesium + oxygen → magnesium oxide. In some reactions, there may be multiple products that need to be separated and purified.
Weather and Climate
What is Weather?
Weather refers to the conditions of the atmosphere at a particular moment and how they change over short periods, such as minutes or days. In some regions, the weather is similar every day, while in others, it varies frequently.
People often ask questions like, "What is the weather like today?" or "Will it rain tomorrow?" Weather is understood as a combination of factors, including temperature, humidity, precipitation, cloud cover, visibility, and wind.
Examples of Different Weather Conditions
- Scotland : Known for its unpredictable weather, experiencing rain, wind, and sunshine all in one day.
- Namibia : Characterized by its hot and dry climate, with vast desert areas and infrequent rainfall.
- Canada : Ranges from cold winters with heavy snowfall to warm summers.
- Republic of Ireland : Features mild but variable weather, often with rain.
- Bangladesh : Tropical climate with hot, humid weather and a distinct monsoon season.
Weather or Climate?
- Weather refers to the short-term conditions in a specific location, while climate is the long-term average of these conditions over a period of about 30 years. Climate includes typical patterns such as average temperature or rainfall.
Climate Zones
The main climate zones on Earth are:
- Polar : Extremely cold and dry throughout the year.
- Temperate : Cold winters and mild summers.
- Arid : Hot and dry all year round.
- Tropical : Hot and humid throughout the year.
- Mediterranean : Mild winters and hot, dry summers.
- Mountains/Tundra/Taiga : Very cold throughout the year.
Climate and Ice Ages
Ice Sheets
During Yuka's time, the Earth was much colder than it is today. A map from 25,000 years ago illustrates the regions covered by large ice sheets.
Glacial and Interglacial Periods
- The cold period when Yuka lived is known as a glacial period, lasting until about 10,000 years ago.
- Today, we are in an interglacial period, characterized by permanent ice near the poles.
- During a glacial period, ice extends further south from the North Pole and further north from the South Pole.
Ice Ages
Scientists have found that the cycle of glacial and interglacial periods did not always occur. There were warm periods without permanent ice on Earth, alternating with cold periods, leading to ice ages.
How Do Scientists Know the Earth Was Colder in the Past?
Some boulders in California indicate they were transported by ancient glaciers. Glaciers move slowly downhill, carrying rocks, and when they melt, these rocks are left behind.
- Occasionally, scientists observe scratches on rocks, suggesting the presence of glaciers.
- In warmer regions, fossils of plants and animals adapted to cold climates provide additional evidence.
Pollen Evidence for Glacial and Interglacial Periods
Scientists analyze pollen to understand ancient plant life. A researcher in New Zealand collected a soil core from a peat bog, where decomposition is slow due to low oxygen levels and acidity.
- The layers of peat represent different historical periods, with deeper layers being older.
- By identifying the types of pollen, scientists can reconstruct past climates.
The Atmosphere and Climate
The Atmosphere
- The atmosphere is the layer of gas surrounding the Earth, which has changed significantly since the planet was formed billions of years ago.
- Scientists believe that Earth was created around 4,600 million years ago, initially being hot and molten. As the planet cooled, volcanic activity released gases, contributing to the formation of the early atmosphere, primarily composed of carbon dioxide.
Earth’s Atmosphere Today
- The modern atmosphere consists of: 78% nitrogen 21% oxygen Traces of carbon dioxide, water vapour, ammonia, and methane
Changes to the Atmosphere
- Around 3,500 million years ago, microorganisms appeared and began using carbon dioxide for food, releasing oxygen as a byproduct. As plants evolved, they also produced oxygen through the process of photosynthesis.
- Over time, the levels of oxygen in the atmosphere increased, leading to its reaction with iron in rocks, forming iron oxide.
Decrease of Carbon Dioxide
- By 200 million years ago, carbon dioxide levels had decreased significantly. Most carbon was used to create essential chemicals in living organisms. Some organisms that do not decompose, such as those that become fossil fuels like oil and coal, trap carbon.
- Between 600 and 400 million years ago, organisms with shells evolved, forming rocks like limestone that also trap carbon.
Atmospheric Changes in Modern Times
- Carbon dioxide levels continued to decrease until about 200 years ago, when they began rising due to human activities.
- Burning fossil fuels such as oil and coal for energy increases carbon dioxide emissions. Deforestation exacerbates the situation by removing trees that absorb carbon dioxide.
Livestock and Their Contribution
- Livestock, including cattle, contribute to carbon dioxide and methane emissions during digestion, further impacting atmospheric changes.
- Processing limestone releases carbon dioxide into the atmosphere, contributing to the increase in carbon levels.
Overall Impact
- These activities lead to significant changes in the atmosphere and an increase in carbon dioxide levels, affecting the balance of gases.
Introduction
 Context Limit Exceeded
Context Limit Exceeded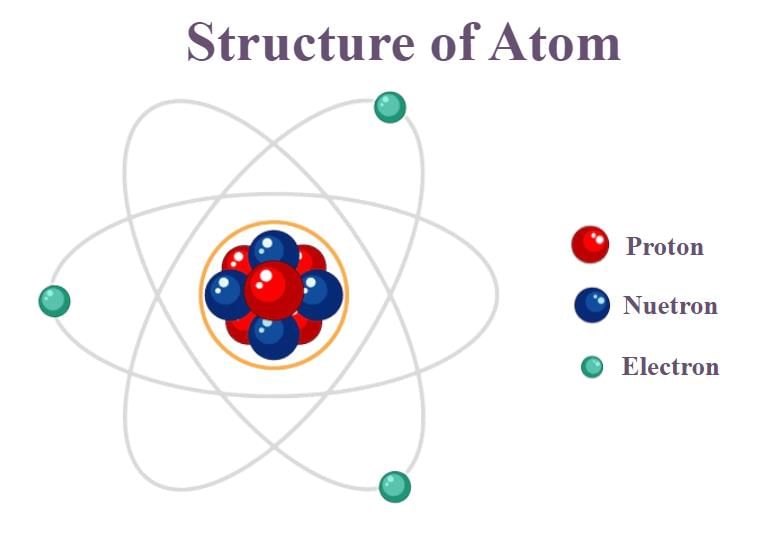
This report delves into the impact of the COVID-19 pandemic on the housing market across various regions in India. It examines how different states responded to the challenges posed by the pandemic and the subsequent changes in housing demand and prices.
Impact of COVID-19 on Housing Demand and Prices

1. Pre-Pandemic Scenario

- Before the pandemic, the housing market in India was witnessing steady growth, with increasing demand and rising prices in many urban areas.
- Factors such as urbanization, low-interest rates, and government initiatives had been driving the housing sector.
2. Pandemic Disruptions

- The onset of COVID-19 in March 2020 led to nationwide lockdowns, disrupting economic activities and causing uncertainty in the housing market.
- Migration patterns changed as people reconsidered their living arrangements, with some moving back to their hometowns while others sought larger homes in less densely populated areas.
3. Regional Variations
a. Maharashtra
- Mumbai: The pandemic initially slowed down sales, but demand for larger homes with better amenities increased as remote work became prevalent.
- Pune: Experienced a surge in demand for residential properties as people sought more space and better living conditions.
b. Delhi-NCR
- Gurugram: Demand for luxury apartments and villas remained strong, with buyers looking for larger spaces.
- Noida: Saw an uptick in demand for affordable housing options, with buyers seeking value for money.
c. Karnataka
- Bengaluru: The tech hub experienced a shift in demand towards suburban areas as remote work became more common.
d. Tamil Nadu
- Chennai: Demand for residential properties in the outskirts increased as families sought larger homes away from the city center.
4. Government Initiatives
- Various state governments introduced measures to boost the housing sector, such as reducing stamp duties and offering incentives for homebuyers.
- These initiatives aimed to stimulate demand and support the recovery of the real estate market.
5. Post-Pandemic Recovery
- As restrictions eased, the housing market showed signs of recovery, with increased sales and renewed interest in residential properties.
- However, the recovery varied by region, with some areas bouncing back more quickly than others.
6. Long-term Changes
- The pandemic has led to lasting changes in housing preferences, with a greater emphasis on space, location, and amenities.
- Buyers are more inclined towards properties that offer a balance of work and leisure, such as homes with dedicated office spaces and access to green areas.
|
3 videos|56 docs|9 tests
|
FAQs on Materials and Their Structure Chapter Notes - Year 8 Science IGCSE (Cambridge)
| 1. What are the basic building blocks of matter? |  |
| 2. How do pure elements differ from compounds? |  |
| 3. What is seawater and what are its main components? |  |
| 4. What is the difference between weather and climate? |  |
| 5. What are glacial and interglacial periods? |  |




















