Matter Chapter Notes | Physics Class 8 ICSE PDF Download
Introduction
In this chapter, we will learn about matter, which is everything around us that has mass and takes up space. We will study the three states of matter—solid, liquid, and gas—and how they behave. We will also understand how matter changes from one state to another, like when ice melts into water or water turns into steam. The chapter explains the tiny particles called molecules that make up matter and how their movement and energy affect the properties of solids, liquids, and gases. By the end, we will know how to observe and describe these changes using simple experiments and scientific ideas.
Matter
Everything around us is made up of matter. Matter is anything that takes up space and has weight. It can be in the form of solids, liquids, or gases.
Composition
- Matter is made up of tiny particles called molecules.
- These tiny particles are very small in size.
- They cannot be seen with our eyes because they are so small.
- Molecules are present in everything around us, like water, air, and even a table.
- These molecules have certain properties that decide whether the matter is a solid, liquid, or gas.
Did You Know?
- Molecules made up of atoms can exist on their own and move freely in nature.
- If a molecule contains just one atom, it is called a monoatomic molecule (like helium, neon, argon, etc.).
- A molecule made of two atoms is known as a diatomic molecule (for example, hydrogen, oxygen, and nitrogen molecules). When a molecule has more than two atoms, it is called a polyatomic molecule (such as water, ammonia, carbon dioxide, etc.).
Characteristics of molecules
Molecules are the tiny particles that make up all matter. They have some special features that help us understand how matter works.
1. They are very small in size:
- Molecules are so tiny that we cannot see them with our eyes.
- The size of a molecule is about 10-10 meters, which is extremely small.
- Even a microscope cannot show us a single molecule because they are too tiny.
2. They have spaces between them:
- The space between molecules is called inter-molecular space.
- In solids, this space is very small because molecules are packed very closely.
- In liquids, the space is a bit more, so molecules are not as close as in solids.
- In gases, the space is the largest, and molecules are very far apart from each other.
- This means solids have the least space, liquids have more, and gases have the most space between molecules.
3. The molecules are in constant motion:
- Molecules are always moving and never stay still.
- This movement happens because they have energy called kinetic energy.
- In solids, molecules vibrate in their fixed positions and do not move around much.
- In liquids, molecules can move more freely within the liquid’s boundaries.
- In gases, molecules move randomly in all directions because they have a lot of space.
4. They attract each other:
- Molecules pull each other with a force called inter-molecular force.
- This force is not because of their weight (like gravity) or their charges (like electric force).
- It is a special force that works only when molecules are very close, up to a distance of 10-9 meters.
- If the distance between molecules becomes more than 10-9 meters, this force disappears.
- In solids, this force is very strong because molecules are very close.
- In liquids, the force is weaker since molecules are farther apart than in solids.
- In gases, the force is very weak, almost not there, because molecules are very far apart.
- The attraction between molecules of the same substance is called the force of cohesion.
- The attraction between molecules of different substances is called the force of adhesion.
- These forces are important because they cause properties like surface tension and viscosity in matter.
Did You Know?
- Liquids and gases can flow because the forces pulling their molecules together are not very strong.
- Adhesives have powerful forces of attraction between their molecules.
States of Matter
- Matter exists in three forms: solid, liquid, and gas.
- For example, ice is a solid, water is a liquid, and steam is a gas.
- Air is also a gas, and oil is a liquid.
- Matter can change its state by changing conditions like temperature.
- Water can exist as ice (solid), water (liquid), and steam (gas) depending on the temperature.
Solids
- Solids are hard and rigid, which means they do not bend or flow easily.
- They have a fixed shape and a fixed volume.
- For example, a book or a stone is a solid with a definite shape and size.
Liquids
- Liquids are not rigid, which means they can flow.
- They have a fixed volume but no fixed shape.
- They take the shape of the container they are put in.
- For example, water in a glass takes the shape of the glass but keeps the same volume.
Gases
- Gases are not rigid and can flow easily.
- They do not have a fixed shape or a fixed volume.
- They take the shape and volume of the container they are in.
- For example, air in a balloon spreads to fill the balloon completely.
Properties That Decide the State of a Substance
Three things decide if a substance is a solid, liquid, or gas. These are:
1. Inter-molecular Space
- This is the space between the molecules of a substance.
- In solids, the space is very small.
- In liquids, the space is more than in solids.
- In gases, the space is the largest.
2. Force of Attraction Between the Molecules
- This is the inter-molecular force that pulls molecules together.
- In solids, this force is very strong.
- In liquids, this force is less strong than in solids.
- In gases, this force is very weak, almost not there.
3. Kinetic Energy of Molecules Due to Their Motion
- Kinetic energy is the energy molecules have because they are moving.
- In solids, the kinetic energy is very small because molecules only vibrate.
- In liquids, the kinetic energy is more because molecules can move around.
- In gases, the kinetic energy is very high because molecules move a lot and in all directions.
Solid
- A substance becomes a solid when the inter-molecular force is very strong.
- At the same time, the kinetic energy of the molecules is very small.
- The inter-molecular space is also very small, so molecules are very close together.
- This is why solids are hard and have a fixed shape.
Liquid
- A substance becomes a liquid when the inter-molecular force is not very strong.
- The kinetic energy of the molecules is enough for them to move around a little.
- The inter-molecular space is more than in solids, so molecules are not as close.
- This is why liquids can flow and take the shape of their container.
Gas
- A substance becomes a gas when the inter-molecular force is very weak, almost not there.
- The kinetic energy of the molecules is very high, so they move a lot.
- The inter-molecular space is very large, so molecules are far apart.
- This is why gases spread out to fill their container completely.
Molecular model of solids
- Solids are made up of very tiny particles called molecules.
- These molecules are very small in size and can be thought of as tiny, hard balls.
- The space between two molecules in a solid, called inter-molecular spacing, is very small.
- Molecules in a solid can only vibrate back and forth around their fixed positions; they cannot move away from their spots.
- The molecules in a solid are very closely packed because of the strong attractive forces between them.
- Because the molecules are in fixed positions and the inter-molecular forces are strong, solids have a definite shape and a definite size (volume).
Molecular model of liquids
- Liquids are made up of very tiny particles called molecules.
- These molecules are very small in size and are not arranged in a fixed or rigid pattern.
- The inter-molecular spaces in liquids are larger than those in solids.
- Molecules in a liquid can move freely within the boundaries of the liquid.
- The molecules in a liquid are less closely packed, and their positions are not fixed because they can move within the liquid’s boundaries.
- This happens because the inter-molecular forces in liquids are weaker compared to those in solids.
- Due to weaker inter-molecular forces, liquid molecules can slide over one another, which allows liquids to flow.
- The inter-molecular forces in liquids, though weak, are strong enough to keep the molecules within the liquid’s boundaries.
- So, liquids have a definite volume but not a definite shape.
Molecular model of gases
- Gases, like solids and liquids, are made up of very tiny particles called molecules.
- These molecules are very small in size and can be thought of as non-rigid balls.
- The separation between molecules in a gas is quite large compared to solids and liquids.
- Molecules in a gas can move freely in all the space available to them.
- The molecules in a gas are far apart, and their positions are not fixed because the inter-molecular forces in gases are very weak.
- Since the inter-molecular forces are so weak, gas molecules are quite free to move here and there in all the space available to them.
- This is why gases do not have a definite shape or a definite volume.
Distinction between solids, liquids, and gases
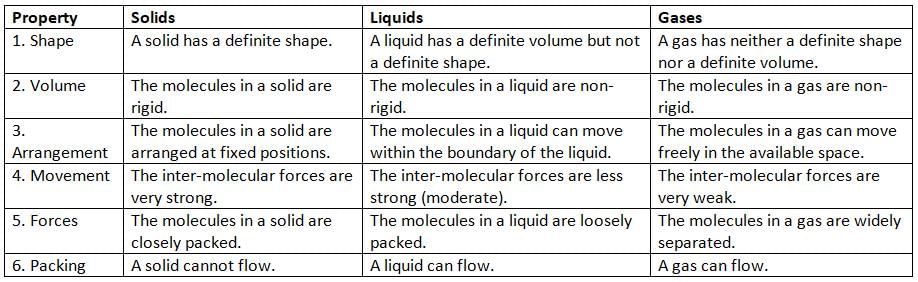
List of few solids, liquids and gases
Change of state
Matter can exist in three forms: solid, liquid, and gas.
When matter changes from one form to another by taking in or giving out heat, it is called a change of state.
Change of Solid into Liquid or Vice-Versa
- When a solid is heated, it turns into a liquid at a certain temperature.
- This process of a solid turning into a liquid is called melting or fusion.
- When the liquid is cooled, it turns back into a solid at the same temperature.
- This process of a liquid turning into a solid is called freezing.
Change of Liquid into Gas or Vice-Versa
- When a liquid is heated, it turns into its gas form, also called vapour, at a certain temperature.
- This process of a liquid turning into a gas is called boiling or vaporization.
- When the gas or vapour is cooled, it turns back into a liquid at the same temperature.
- This process of a gas turning into a liquid is called condensation.
Change of Solid into Gas or Vice-Versa
- In some special cases, like with camphor or iodine, a solid directly turns into its gas form when heated, without becoming a liquid first.
- This process of a solid turning directly into a gas is called sublimation.
- When the gas is cooled, it turns back into a solid directly, without becoming a liquid.
- This process of a gas turning directly into a solid is called deposition.
Melting
- When a solid is heated at a certain temperature, it changes into a liquid.
- This process is called melting.
- The temperature at which a solid turns into a liquid without any further increase in temperature is called the melting point of the solid.
- For example, ice, which is a solid, melts at 0°C to form water, which is a liquid, by taking in heat.
Freezing
- When a liquid is cooled at a certain temperature, it changes into a solid.
- This process is called freezing.
- The temperature at which a liquid turns into a solid without any further decrease in temperature is called the freezing point of the liquid.
- For example, water, which is a liquid, freezes at 0°C to form ice, which is a solid, by giving out heat.
Note:
- The changes in the state of matter happen at a fixed temperature.
- During these changes, the heat given to or taken from the substance does not cause a change in its temperature.
- This heat is called latent heat, which means hidden heat, because it does not show as a temperature change.
- The melting and freezing processes can be shown using powdered naphthalene or solid ice.
- For any substance, the melting point and freezing point are the same.
- For example, the melting point of wax is 55°C, and the freezing point of melted wax is also 55°C.
Melting point and heat absorbed during melting for some substances
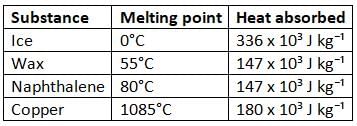
Do you know?
- The amount of heat needed during melting of a substance is called the latent heat of fusion.
- The amount of heat absorbed or released during melting is the same for a substance.
- The amount of heat absorbed or released during melting of a substance is measured in joule per kilogram.
- Wax expands on freezing whereas water, lead, etc., contract on freezing.
- Melting point of ice decreases with increase of pressure on it, but the melting point of wax increases with pressure on it.
- Melting point of ice decreases when salt is added to ice.
- This mixture is used for preparing freezing mixture.
Explanation of melting by molecular model
- In a solid, the molecules are very close to each other and tightly packed.
- The molecules in a solid only vibrate in their positions because they have less energy.
- They have strong forces of attraction holding them together.
- When we heat a solid, the kinetic energy of the molecules increases, and they start to vibrate more.
- At a certain temperature called the melting point, the molecules gain enough energy to overcome the forces of attraction.
- The molecules then move apart from each other and become free to move within the substance.
- In this state, the heat energy absorbed by the substance does not change the temperature but is used to overcome the forces of attraction between the molecules.
- This increases the potential energy of the molecules (their energy due to position).
Vaporization or boiling
- This is the process where a liquid turns into a gas (vapor) by absorbing heat at a constant temperature.
- The specific temperature at which this happens, without any further temperature increase, is called the boiling point.
- For example, water (a liquid) boils at 100°C at standard atmospheric pressure, turning into steam (a gas) by absorbing heat while staying at 100°C during the process.
Condensation
- This is the reverse process, where a gas (vapor) turns back into a liquid by releasing heat (or cooling) at a constant temperature.
- The temperature at which this occurs, without any further decrease in temperature, is called the condensation point.
- For example, steam at 100°C condenses back into water (liquid) at 100°C by releasing heat during cooling.
Boiling point and heat absorbed during vaporization of some liquids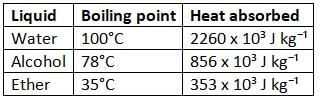
Do you know?
- For a substance, the boiling point and condensation point are the same. For example, boiling and condensation of water happen at 100°C.
- The amount of heat absorbed or rejected during the change from liquid to vapor state (or vapor to liquid) is called the latent heat of vaporization.
- All liquids expand on heating.
- The boiling point of a liquid increases with the increase of pressure on it. This is why it is difficult to cook vegetables at mountainous regions where pressure is low and boiling point is low, but it is easy to cook vegetables in a pressure cooker where pressure is high and boiling point is increased by keeping the vapor inside the cooker.
- The boiling point of a liquid decreases when impurities are added to it.
Explanation of vaporization by molecular model
- In a liquid, the molecules can move in all directions but are still close to each other.
- The molecules in a liquid have small forces of attraction holding them together.
- They have low kinetic energy (energy of movement).
- When we heat a liquid, the kinetic energy of the molecules increases.
- At a particular temperature called the boiling point, the molecules gain enough energy to overcome the forces of attraction.
- The molecules then become free to move and leave the liquid surface.
- In this state, the heat energy absorbed by the liquid does not change the temperature but is used to overcome the forces of attraction between the molecules.
- This increases the potential energy of the molecules (their energy due to position).
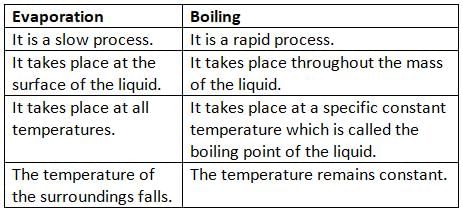
Evaporation
- Evaporation is another process where a liquid changes into vapor (gas).
- The change of state from the surface of a liquid is called evaporation.
- Evaporation happens at all temperatures, but vaporization (boiling) happens only at a fixed temperature called the boiling point.
- Evaporation is a slow and gradual process, while vaporization is a fast and sudden process.
- Evaporation happens only at the surface of the liquid, but vaporization happens throughout the entire liquid.
- Evaporation has a cooling effect, but vaporization does not produce a cooling effect.
Rate of Evaporation
The speed at which a liquid turns into gas is called the rate of evaporation. It depends on five main things:
- Temperature of Liquid: A wet cloth dries faster on a hot day than on a cold day. When the temperature of the liquid is higher, it evaporates faster.
- Area of the Exposed Surface: A cloth dries faster when it is spread out than when it is folded. If the surface area of the liquid that is open to air is bigger, the rate of evaporation increases.
- Nature of Liquid: Some liquids, like alcohol and spirit, evaporate faster than water. This is because these liquids are volatile, which means they turn into gas easily. That’s why we store volatile liquids in tightly closed bottles.
- Flow of Air Above the Liquid: If air moves over the surface of a liquid, the liquid turns into gas faster. The moving air takes away the gas particles from the surface of the liquid. This is why we blow air over hot milk to cool it down faster.
Presence of Humidity: Air with more water vapor in it is called humid air. In humid air, evaporation happens slower than in dry air. This is why wet clothes dry faster on dry summer days than on rainy days.
Explanation of Evaporation by the Molecular Model
- Liquids are made of tiny particles called molecules.
- Inside a liquid, these molecules are always moving and bumping into each other.
- Some molecules near the surface get enough energy to overcome the pull from other molecules.
- These molecules then escape into the air as gas.
- This process of molecules escaping from the liquid surface into the air is called evaporation.
Did You Know?
- Higher liquid temperatures lead to more molecules escaping from its surface.
- A larger exposed surface area of the liquid results in more molecules escaping.
- In volatile liquids, the weak attraction between molecules allows them to escape more easily.
- Blowing air over a liquid removes liquid molecules from the surface, along with other molecules, increasing the evaporation rate.
- In humid air, water molecules in the air above the liquid hinder the liquid molecules from escaping, reducing the evaporation rate.
Evaporation Produces Cooling
- When a liquid like alcohol or spirit is put on your palm, it feels cool.
- Similarly, when water is poured on a hot surface, the surface cools down.
- During evaporation, the liquid takes heat from its surroundings to change into gas.
- This heat is used by the liquid’s molecules to gain energy and escape as gas.
- Because the liquid takes heat away, the surroundings, like your palm or the surface, become cooler.
- This is why we feel cool when we sweat on a hot day—the sweat evaporates and takes heat from our body.
Applications of Evaporation
- In summer, water gets cooled in an earthen pot (called a surahi).
- The pot has tiny holes, and water comes out through these holes to the surface.
- The water on the surface evaporates, taking heat from the water inside the pot, making it cool.
- Doctors tell us to put wet cloth strips on the forehead of a patient with high fever.
- The water in the strips evaporates, taking heat from the patient’s forehead, which helps lower the fever.
- We often pour tea into a saucer to cool it faster.
- In the saucer, the tea has a larger surface area, so it evaporates faster and cools down quickly.
- During summer, we wear cotton clothes because they help sweat evaporate faster.
- Sweat takes heat from our body to evaporate, which helps keep our body temperature at 37°C.
- When sweat evaporates, it takes heat away from our body, making us feel cooler.
Difference Between Evaporation and Boiling
Sublimation and Deposition
- Sublimation is when a solid directly changes into a gas without becoming a liquid first.
- For example, camphor and naphthalene change from solid to gas when heated.
- Iodine and solid carbon dioxide (dry ice) also change directly from solid to gas when heated.
- Deposition is the opposite—it’s when a gas directly changes into a solid without becoming a liquid.
- For example, ammonium chloride gas cools and turns into a solid on a cool surface.
Examples of Sublimation and Deposition
- Some solids, like naphthalene balls or moth balls, slowly change into gas to keep insects away.
- We use these in woolen clothes to protect them from insects during storage.
- The process of a gas turning directly into a solid is called deposition.
- For example, ammonium chloride gas cools on a surface and becomes a solid again.
Explanation of Sublimation by the Molecular Model
- In some solids, the tiny particles (molecules) are held together by weak forces of attraction.
- When we heat these solids, the molecules get enough energy to overcome these weak forces.
- The molecules then break free and turn directly into a gas—this is called sublimation.
- When the gas cools down, the molecules lose energy and come closer together again.
- They form a solid directly without becoming a liquid—this is called deposition.
Did You Know?
- Ice at 0°C absorbs 336 × 10³ joules per kilogram of heat to change into water at 0°C without changing its temperature.
- The change from the solid state to the liquid state at a fixed temperature is called melting.
- The heat absorbed by a solid during melting without a change in temperature is called its latent heat of fusion.
- Water at 100°C absorbs 2260 × 10³ joules per kilogram of heat to change into steam at 100°C without changing its temperature.
- Steam at 100°C changes into water at 100°C by giving out 2260 × 10³ joules per kilogram of heat.
- The change from the liquid state to the gas state at a fixed temperature is called boiling.
- The heat absorbed by a liquid during boiling at a fixed temperature is called its latent heat of vaporization.
- The heat absorbed by a liquid during vaporization and the heat given out by a gas during condensation are the same for the same substance.
Points To Remember
- Anything that takes up space and has mass is called matter.
- All things around us are made of tiny particles called molecules.
- Molecules are so small that they can only be seen with special microscopes.
- A molecule is made of two or more atoms joined together.
- Molecules in matter always have some motion, which can be moving in a straight line, spinning, or vibrating.
- The molecules in a substance are held together by a force called the force of attraction.
- The force of attraction is different for different types of substances—it’s called the force of cohesion when it’s between molecules of the same kind.
- The force of attraction between molecules of different substances is called the force of adhesion.
- In solids, the space between molecules is very small, so the force of attraction is strong.
- In liquids, the space between molecules is larger than in solids, so the force of attraction is weaker.
- In gases, the space between molecules is very large, so the force of attraction is very weak.
- In solids, the force of attraction between molecules is the strongest, in liquids it’s medium, and in gases it’s the weakest.
- The process of a solid changing into a liquid is called melting, and the temperature at which this happens is called the melting point.
- The process of a liquid changing into a gas at a fixed temperature is called boiling, and the temperature at which this happens is called the boiling point.
- The process of a liquid changing into a gas at all temperatures from its surface is called evaporation.
- Evaporation is a slow process and depends on the temperature of the liquid, the area of the surface exposed to air, the type of liquid, the flow of air above the liquid, and the amount of humidity in the air.
- Sublimation is when a solid directly changes into a gas, like camphor or naphthalene does when heated.
- Deposition is when a gas directly changes into a solid, like ammonium chloride does when it cools.
|
8 videos|40 docs|8 tests
|
FAQs on Matter Chapter Notes - Physics Class 8 ICSE
| 1. What is matter, and how is it classified? |  |
| 2. What are molecules, and how do they differ from atoms? |  |
| 3. What are the characteristics of molecules? |  |
| 4. How do temperature and pressure affect the state of matter? |  |
| 5. Why is understanding matter and its properties important in our daily life? |  |
















