Metabolism of Lipids - 1 Chapter Notes | Chemistry for ACT PDF Download
| Table of contents |

|
| Digestion and Absorption of Lipids |

|
| Metabolism of Triacylglycerol |

|
| Lipolysis in Adipose Tissue |

|
| Metabolism of Fatty Acids |

|
| Cholesterol |

|
Digestion and Absorption of Lipids
The primary lipids in our diet are triacylglycerols (more than 90%), with the rest consisting of phospholipids and cholesterol.
Enzymes Involved in Lipid Digestion
Lingual Lipase and Gastric Lipase
- Lingual and gastric lipases initiate the breakdown of triacylglycerols.
- Location: Stomach
- Function: Hydrolyzes the third ester bond of triacylglycerol.
- Digestive Contribution: 30% of total triacylglycerol digestion occurs in the stomach.
Pancreatic Lipase
- Colipase, another pancreatic protein, is necessary for activating pancreatic lipase.
- Colipase plays a crucial role in preventing bile from inhibiting lipases.
- Pancreatic lipase is the primary enzyme responsible for breaking down triacylglycerols.
- The hydrolysis of triacylglycerol primarily occurs at positions 1 and 3 of the glycerol backbone.
- This enzyme is vital for digesting dietary fats as it helps in the breakdown of fat molecules.
- Additionally, pancreatic lipase acts on various lipid substrates, facilitating improved fat absorption.
Pancreatic Esterase
- Pancreatic esterase is responsible for breaking down approximately 25% of monoacylglycerols into glycerol and fatty acids.
- Key substrates for pancreatic esterase include cholesterol esters and other lipid esters, which are essential for lipid digestion.
Phospholipases
- The document contains several sections with unclear or corrupted text.
- Symbols and characters should be replaced with clear text to enhance understanding.
- Readable content is essential for effectively conveying the message.
Absorption of Lipids
Role of Bile Salt/Bile Acids
- The document has numerous unclear sections filled with corrupted text.
- These sections should be replaced with comprehensible text for improved clarity.
- At normal body pH, bile acids are predominantly in an ionized form (anions).
- The products of lipid digestion are hydrophobic.
- Bile acids assist in transporting digestion products through the aqueous environment of the intestinal lumen.
- This process enables these products to reach the brush border of the mucosal cells for absorption into epithelial cells.
- Fat-soluble vitamins, including A, D, E, and K, also require bile acids for their absorption.
Inside Intestinal Epithelial Cells
- Glycerol, released in the intestinal lumen, is absorbed into the bloodstream for further metabolic processing.
- Chylomicrons are secreted into the lymphatics and subsequently enter the bloodstream.
- Monoacylglycerols are converted back to triacylglycerols through the monoacylglycerol pathway.
- The newly synthesized triacylglycerol, along with cholesterol esters and phospholipids, is added to chylomicrons.
- Chylomicrons are then secreted into the lymphatics, from where they enter the bloodstream.
Metabolism of Triacylglycerol
Synthesis of Triacylglycerol
Triacylglycerol is the primary form of simple lipids. It is produced in almost all body tissues, with the liver being the most significant site of production. The majority of triacylglycerol synthesis occurs in the endoplasmic reticulum, although some synthesis also takes place in the mitochondria.
Three Steps for TAG Synthesis
- Step 1: Acylation of glycerol-3-phosphate.
- Step 2: Formation of diacylglycerol.
- Step 3: Synthesis of triacylglycerol from diacylglycerol.
Activation of Fatty Acids
- Enzyme. Acyl CoA Synthetase or Thiokinase
- Reaction. Fatty acid + CoA + ATP → Acyl-CoA + AMP + PPi
Activation of Glycerol
- Enzyme: Glycerol Kinase
- Reaction: Glycerol is phosphorylated to form Glycerol 3-phosphate.
In Muscle and Adipose Tissue
- Glycerol Kinase is absent in muscle and white adipose tissues.
- This absence prevents the use of glycerol for gluconeogenesis in these tissues.
Synthesis of Triacylglycerol in the Intestine - Monoacylglycerol Pathway
- Enzyme: Acyltransferase
- Reaction: Transfer of 3 acyl moieties to glycerol
Synthesis of Triacylglycerol in the Intestine - Monoacylglycerol Pathway
- In the intestinal mucosa, monoacylglycerol transferase plays a crucial role in converting monoacylglycerol into 1,2-diacylglycerol through the monoacylglycerol pathway.
Degradation of Triacylglycerol (Lipolysis)
- Triacylglycerols are broken down by a lipase into their individual components.
- Most of this breakdown (lipolysis) happens in adipose tissue.
- They are used in various tissues including the heart, kidney, muscle, lung, testis, and adipose tissue, but are not easily used by the brain.
- The use of glycerol depends on the presence of the enzyme glycerol kinase in tissues.
- Glycerol can be used in the liver, kidney, intestine, brown adipose tissue, and some other tissues.
- NB: Brown adipose tissue has glycerol kinase, while white adipose tissue does not.
Lipolysis in Adipose Tissue
Lipolysis is the process of breaking down triglycerides (TAG) stored in fat tissue. This process is carried out by an enzyme called hormone-sensitive lipase (hSL). During lipolysis, hSL acts on the TAG by transforming the first and third carbons into diacylglycerol and releasing a fatty acid from the second carbon. The released fatty acid is then removed by 2-acylglycerol.
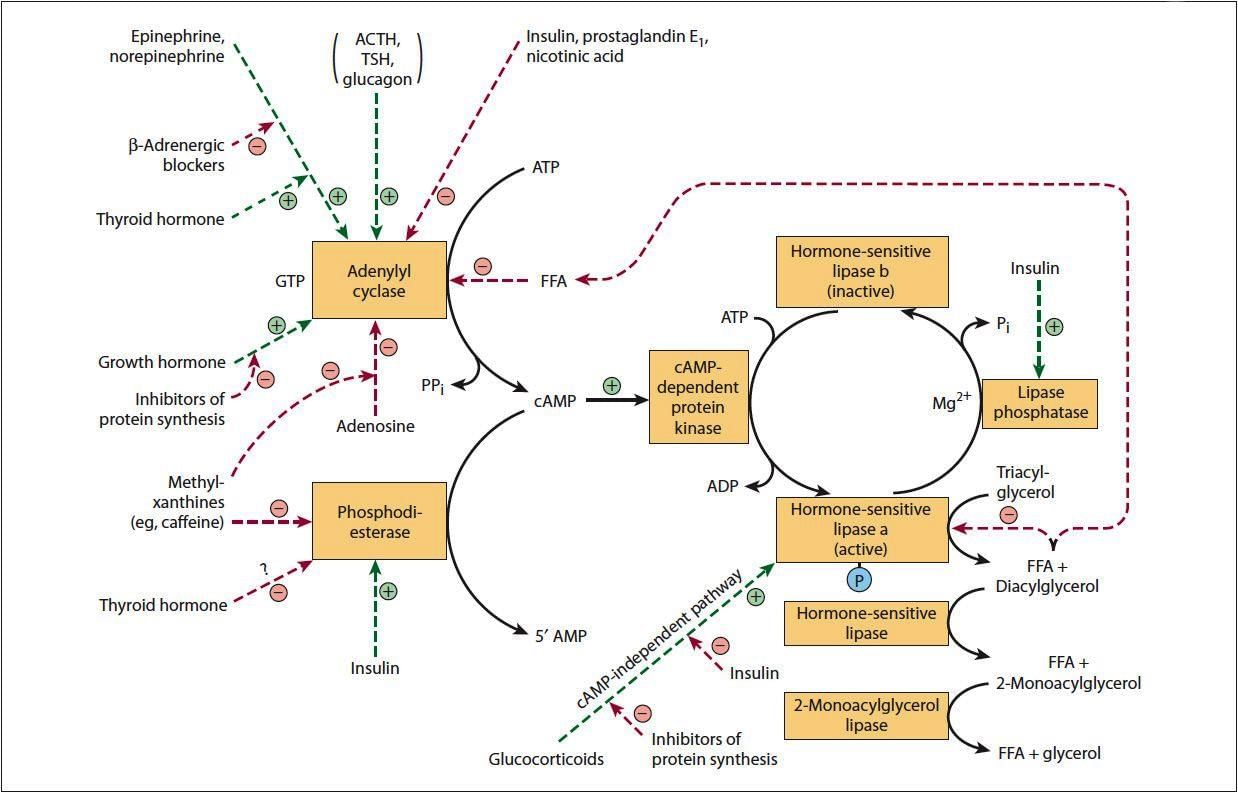
Regulation of Hormone-Sensitive Lipase
- The activity of hormone-sensitive lipase is regulated by hormones.
- The enzyme is active when it is in a phosphorylated state.
- Various hormones and their signaling pathways control the regulation of hSL.
Hormone-sensitive Lipase Activation
- Glucagon
- Catecholamines (Epinephrine and Norepinephrine)
- ACTH
- TSH
- Glucocorticoids
- Thyroid hormones
- Growth hormone
Mechanism of Activation
- Hormone-sensitive lipase is activated by the stimulation of adenylyl cyclase, leading to an increase in cAMP levels.
- The rise in cAMP activates protein kinase A, which phosphorylates and activates hormone-sensitive lipase.
- Glucocorticoids, such as cortisol, promote lipolysis by enhancing the expression of specific lipolytic enzymes.
Factors That Inactivate Hormone Sensitive Lipase
- Insulin
- Nicotinic Acid
- Other factors
Mechanism of Inactivation of Hormone Sensitive Lipase
- Prostaglandin E1 inhibits lipase activity, which is regulated by insulin.
- Insulin enhances phosphodiesterase, leading to the inactivation of hormone-sensitive lipase.
Metabolism of Fatty Acids
De Novo Fatty Acid Synthesis (Lipogenesis)
- This process is commonly referred to as Lynen’s Spiral.
- Significant sites for fatty acid synthesis include the liver, kidneys, brain, lungs, and lactating mammary glands.
- The synthesis occurs through an extramitochondrial system, outside of the mitochondria.
- Essential cofactors for this process include CoA, Biotin, and HCO3–, which provides carbon dioxide (CO2).
- Acetyl-CoA plays a crucial role in various biochemical reactions within metabolic pathways, acting as a key intermediary.
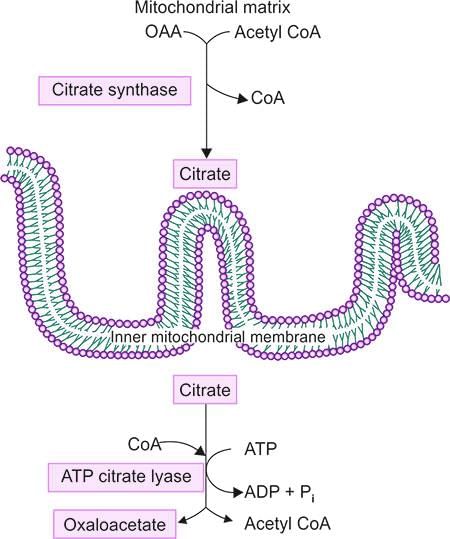
Sources of Acetyl CoA
- Acetyl CoA is a crucial molecule in metabolism, playing a role in various biochemical processes.
- It is primarily derived from the breakdown of carbohydrates, fats, and proteins.
Transport of Acetyl CoA
- Acetyl CoA cannot cross cellular membranes by itself; it requires specific transport proteins for its movement.
- These proteins facilitate the transfer of Acetyl CoA into the mitochondria, where it enters the citric acid cycle.
- Within the mitochondria, Acetyl CoA reacts with oxaloacetate to form citrate.
- Citrate is then transported into the cytosol through the Tricarboxylate Transporter.
- In the cytosol, citrate is cleaved by the enzyme ATP Citrate Lyase into acetyl-CoA and oxaloacetate.
By Two Enzyme System
Acetyl CoA Carboxylase
- Acetyl CoA carboxylase is an enzyme that converts Acetyl CoA (a 2-carbon molecule) into Malonyl CoA in the cytosol.
- This enzyme requires vitamin B7, also known as biotin, to function properly.
- The conversion of Acetyl CoA to Malonyl CoA by this enzyme is the rate-limiting step in the metabolism of fatty acids.
- It is important to note that Acetyl CoA carboxylase is not a part of the fatty acid synthase complex.
Fatty Acid Synthase (FAS) Multienzyme Complex
- The fatty acid synthase complex is made up of two identical polypeptide chains, forming a homodimer.
- It contains vitamin Pantothenic acid in the form of 4’-phosphopantotheine.
- X-ray crystallography studies have shown that the complex has an X-shaped structure.
Sources of Fatty Acid Synthases
- Ketoacyl Synthase
- Malonyl-Acetyltransacylase
- Hydratase
- Enoyl Reductase
- Ketoacyl Reductase
- Thioesterase (Deacylase)
Sources of NADPH
- NADPH can be generated through various metabolic pathways, including the pentose phosphate pathway and the activity of the malic enzyme.
- The malic enzyme facilitates the conversion of malate to pyruvate, a process that produces NADPH.
- Additionally, the mitochondrial enzyme isocitrate dehydrogenase contributes to NADPH generation by oxidizing isocitrate.
Stages of Reactions in Fatty Acid Synthase Complex
The stages are:
(i) Condensation
(ii) Reduction
Condensation Reaction
- Malonyl/Acetyl Transacylase: This is the initial step in the process of synthesizing fatty acids.
- Formation of Malonyl-Acetyl Enzyme: When malonyl-CoA combines with an acetyl group, it produces the malonyl-acetyl (acyl) enzyme.
- Role of Malonyl-Acetyl Transacylase: This enzyme facilitates the reaction, leading to the formation of the acetyl (acyl)-malonyl enzyme.
- Ketoacyl Synthase: In the next step, the acetyl group interacts with the methylene part of the malonyl residue. This reaction is catalyzed by ketoacyl synthase.
- Formation of 3-Ketoacyl Enzyme: During this process, carbon monoxide (CO) is released, and the 3-ketoacyl enzyme (also known as the acetoacetyl enzyme) is formed.
Reduction Reactions in Fatty Acid Synthesis
- The 3-ketoacyl group undergoes reduction to form a hydroxyacyl group, facilitated by the enzyme ketoacyl reductase.
- Subsequently, the hydroxyacyl group is transformed into an unsaturated acyl (enoyl) group through the action of hydratase.
- The unsaturated acyl (enoyl) group is then reduced to the corresponding acyl group by the enzyme enoyl reductase.
Fatty Acid Synthesis Complex
- The processes of condensation and reduction are repeated multiple times until the desired acyl group is assembled on the enzyme.
- Once assembled, the acyl group is transferred to the acyl carrier protein complex by the enzyme thioesterase (deacylase), the sixth enzyme in the complex.
Regulation of Fatty Acid Synthesis
- Rate Limiting Step: The regulation of fatty acid synthesis is primarily controlled at the level of Acetyl CoA Carboxylase, which is the rate-limiting enzyme in the process.
Long Term Regulation
Long term regulation of enzyme activity involves the control of enzyme synthesis by regulating gene expression.
Short Term Regulation
- Allosteric regulation involves the regulation of enzymes through the binding of molecules at sites other than the active site, leading to conformational changes in the enzyme.
- Regulation of Acetyl CoA Carboxylase by citrate. Citrate plays a crucial role in activating Acetyl CoA Carboxylase by converting it from an inactive dimer to its active form.
Inactivation Mechanism. Long-chain Acyl CoA induces inactivation by:
- Inhibiting the tricarboxylic transporter, which is responsible for transporting Acetyl CoA into the mitochondria.
Regulation of Enzyme Activity
- Enzyme activity can be regulated by adding or removing phosphate groups through hormonal action.
- Acetyl CoA Carboxylase is in an active state when it is dephosphorylated.
Glucagon and Epinephrine
- Glucagon and epinephrine play a role in inactivating Acetyl CoA.
Mechanism of Action of Glucagon and Epinephrine
- Glucagon and epinephrine trigger the breakdown of glycogen into glucose in the liver, leading to an increase in blood glucose levels.
- These hormones also promote gluconeogenesis, the process of generating glucose from non-carbohydrate sources.
- Furthermore, glucagon and epinephrine enhance lipolysis, which is the breakdown of fats into fatty acids for energy.
- Glucagon and epinephrine are essential for maintaining energy balance and responding to stress by increasing the availability of glucose and fatty acids for metabolism.
- Insulin activates Acetyl CoA Carboxylase by dephosphorylating the enzyme, promoting the conversion of Acetyl CoA into malonyl CoA, a key step in fatty acid synthesis.
How Insulin Works
- When insulin is active, it removes a phosphate group from Acetyl CoA carboxylase.
Acetyl CoA carboxylase plays a crucial role in various metabolic processes.
- Phosphorylation refers to the addition of a phosphate group, which can inactivate acetyl CoA carboxylase.
- Dephosphorylation, on the other hand, removes the phosphate group and activates the enzyme.
- Hormones like glucagon and epinephrine promote the phosphorylation and inactivation of acetyl CoA carboxylase.
Fate of Acyl CoA
Acyl CoA can undergo several processes, including:
- Chain elongation, leading to the formation of very long chain fatty acids.
- Desaturation, which produces unsaturated fatty acids.
These processes involve different metabolic pathways that influence the destiny of fatty acids.
Elongation of Fatty Acid Chains
- Location: The elongation of fatty acid chains occurs in the Endoplasmic Reticulum (ER).
- Role of Malonyl CoA: Malonyl CoA gradually provides two carbon atoms for the elongation process.
- Starting Point: Elongation begins with fatty acids C10 and involves the addition of two carbon atoms.
- Increased Elongation in the Brain: This process is particularly enhanced in the brain during myelination, leading to the production of C22 and C24 fatty acids.
Synthesis of Unsaturated Fatty Acids
- Humans cannot add additional double bonds to fatty acids.
- Some polyunsaturated fatty acids are not produced by the human body, but they can be obtained through dietary sources.
- Providing a clearer statement would enhance the understanding of fatty acid synthesis.
Oxidation of Fatty Acids
- Fatty acids undergo oxidation processes to generate energy.
- This involves various biochemical pathways, with the β-oxidation pathway being essential for the metabolism of fatty acids in the body.
Activation of Fatty Acids
- The activation of fatty acids occurs in the cytoplasm, where two carbons are sequentially removed from the carboxyl end of the fatty acid chain.
- This process is facilitated by various enzymes, with Acyl CoA Synthetase playing a crucial role in activating fatty acids.
- The primary organs involved in this process are the Liver, Adipose Tissue, and Muscle.
- The organelle responsible for fatty acid activation is the Mitochondria.
Fatty Acid Metabolism
- After activation, fatty acids are further metabolized in the mitochondria through a process known as beta-oxidation.
- During beta-oxidation, fatty acids are broken down into acetyl CoA units. These acetyl CoA units then enter the Krebs cycle, where they are utilized for energy production.
- This metabolic pathway is essential for maintaining energy homeostasis in the body and is regulated by various physiological factors.
Fatty Acid Activation Process
- Site: Cytoplasm
- Enzyme: Acyl CoA Synthetase
- During the activation of fatty acids, two inorganic phosphates are released.
Transport of Acyl CoA into Mitochondria
- Acyl CoA cannot pass through the mitochondrial membrane directly.
- It must first be changed into acylcarnitine by the enzyme carnitine acyltransferase I, found on the outer mitochondrial membrane.
- After forming acylcarnitine, it is moved across the inner mitochondrial membrane by carnitine acylcarnitine translocase.
- Once inside the mitochondria, acylcarnitine is converted back to Acyl CoA by the enzyme carnitine acyltransferase II.
Carnitine acyltransferase I
- This enzyme transfers the acyl group from Acyl CoA to carnitine, creating acylcarnitine.
- Acylcarnitine is then moved across the inner mitochondrial membrane.
Carnitine acyltransferase II
- This enzyme is located in the inner mitochondrial membrane and changes acylcarnitine back to Acyl CoA.
Reactions Involved in Beta-Oxidation
Beta-oxidation is a process that involves a sequence of four reactions, which progressively remove 2-carbon units from fatty acids to produce Acetyl CoA.
Production of Reducing Equivalents
- Acyl-CoA Dehydrogenase: This enzyme initiates the beta-oxidation process by removing hydrogen atoms from the acyl-CoA molecule, leading to the formation of trans-Δ 2-enoyl-CoA and generating FADH 2 .
- Hydration: The next step involves the addition of a water molecule to the trans-Δ 2-enoyl-CoA, resulting in the formation of L-3-hydroxyacyl-CoA.
- Enoyl CoA Hydratase: This enzyme facilitates the hydration reaction, converting trans-Δ 2-enoyl-CoA into L-3-hydroxyacyl-CoA.
- Hydroxy Acyl CoA Dehydrogenase: In this step, L-3-hydroxyacyl-CoA is oxidized to 3-ketoacyl-CoA by the enzyme hydroxy acyl CoA dehydrogenase, producing NADH in the process.
- Cleavage: The 3-ketoacyl-CoA is then cleaved, releasing Acetyl CoA and a new acyl-CoA molecule that is two carbon atoms shorter than the original.
- Thiolase: This enzyme catalyzes the final step of beta-oxidation, where the acyl-CoA is cleaved to release acetyl-CoA and a new acyl-CoA molecule, continuing the cycle.
Overall Recommendations
- Focus on correcting spelling and grammatical errors.
- Ensure clarity in sentence structure.
- Verify factual accuracy.
- Maintain a consistent and professional tone throughout.
- Format the document with clear headings.
- Avoid using non-standard symbols.
Beta-Oxidation of Palmitic Acid (C-16)
- In the process of beta-oxidation, palmitic acid (C-16) is broken down into acetyl CoA units, where n represents the number of carbon atoms.
- Each cycle of beta-oxidation shortens the fatty acid chain by two carbon atoms, producing one molecule of acetyl CoA and generating ATP and NADH.
- For palmitic acid, the total ATP yield can be calculated as follows:
- For each molecule of palmitic acid, the complete oxidation results in 106 ATPs.
- Calculate the number of 2-carbon Acetyl CoA molecules derived from 16-carbon palmitic acid using the formula (n/2), where n is the number of carbon atoms: (16/2) = 8 Acetyl CoA molecules.
- The total yield from the complete oxidation of palmitic acid is 80 ATPs.
- Thus, the net ATPs calculated from palmitic acid are 108 – 2 = 106 ATPs.
From Stearic Acid (18C)
The net ATPs produced from stearic acid are 122. 2. 120 ATPs.
Controlled by CPT-I Gateway
In the Fed State
- Acetyl CoA Carboxylase is increased.
- Levels of Malonyl CoA are elevated.
- Regulation of Fatty Acid Metabolism is taking place.
In the Fasting State
- Insulin/Glucagon ratio is decreased.
- Activity of Acetyl CoA Carboxylase is reduced.
- Malonyl CoA levels do not increase.
- Regulation of Beta oxidation of fatty acids is conceptually understood.
- During fasting, ATP production primarily occurs through the oxidation of fatty acids.
Clinical Connections
Primary Clinical Indicators
Metabolic disorders in infants can present a range of symptoms, including:
- Hypoglycemia during fasting
- Absence of ketone bodies
- Symptoms such as vomiting, coma, and potentially death
- Presence of C8-C10 acylcarnitine in the blood
- Episodes may be triggered by an overnight fast in infants
- Initial treatment involves intravenous glucose
- Metabolic imbalance can lead to severe consequences if not promptly addressed
Additional Considerations
- Impairment of fatty acid oxidation due to Medium-Chain Acyl-CoA Dehydrogenase (MCAD) deficiency can result in significant metabolic problems
- Understanding the underlying biochemical pathways is essential for accurate diagnosis and effective treatment
What is a Dataset?
- A dataset is a collection of data that is organized in a specific way, usually in rows and columns, similar to a table. Each row represents a single observation or record, while each column represents a specific variable or feature of that data. Datasets are commonly used in various fields such as research, business, and machine learning to analyze and draw insights from data.
Clinical Features
- Hypoglycemia: Low blood sugar due to impaired glucose processing.
- Muscular Weakness: Caused by fat accumulation in muscles.
- Dietary Requirement: Similar to vitamin needs.
- Treatment: Carnitine supplements recommended.
Clinical Implications
- Can lead to serious metabolic disturbances requiring careful management.
Management Strategies
- Involves dietary changes and regular monitoring of nutrient intake.
Sulfonylurea in Type II Diabetes Mellitus
- Sulfonylurea drugs, including glyburide (also known as glibenclamide. and tolbutamide, are commonly used to treat type 2 diabetes mellitus.
- These medications work by inhibiting insulin secretion, which in turn helps to reduce gluconeogenesis and prevent hyperglycemia.
Defects in Long Chain 3-Hydroxyacyl-CoA Dehydrogenase
- The process of fatty acid oxidation begins in the peroxisomes, where Octanoyl CoA is produced.
- During this oxidation process in the peroxisomes, Acetyl CoA and H2O2 are generated as byproducts.
- Subsequently, fatty acid oxidation continues in the mitochondria, where the enzymes responsible for this process are located in the mitochondrial matrix.
Ketogenesis
- Ketogenesis is a metabolic process that occurs in the liver and is regulated by the levels of fatty acids and acetyl CoA.
- This pathway becomes particularly important during periods of fasting, low-carbohydrate diets, or prolonged exercise when the body needs to generate alternative energy sources.
- However, if ketogenesis is not properly regulated, it can lead to serious conditions such as diabetic ketoacidosis.
Defects in the Oxidation of Very Long-Chain Fatty Acids (VLCFA) in the Peroxisomes
Peroxisomal diseasesare genetic disorders that occur due to:
- Inability to produce or maintain peroxisomes.
- Dysfunction of a specific enzyme within the peroxisome.
Basic Defect in Peroxisomal Diseases
- Proteins destined for peroxisomes play crucial roles in various metabolic processes.
- These proteins are vital for:
- Decomposing fatty acids.
- Clearing out toxic substances.
Peroxisomal Ghost in Disorders
- A hallmark of peroxisomal disorders is the absence or significant reduction of peroxisomes.
- In most of these disorders, there are membranous sacs that contain:
Peroxisomal integral membrane proteins.
- However, these sacs are deficient in the normal array of matrix enzymes required for effective metabolic functioning.
Peroxisomal Disorders
- Zellweger’s syndrome
- Neonatal adrenoleukodystrophy (NALD)
- Infantile Refsum disease (IRD)
- Adrenoleukodystrophy
Lorenzo’s Oil Therapy
- Adrenoleukodystrophy is treated with Lorenzo’s oil, a mixture of glyceryltrioleate and glyceryltrierucate in a 4:1 ratio.
Fatty Acid Metabolism
- Fatty acid metabolism involves several steps, starting with the breakdown of fatty acids through beta-oxidation.
- Normal beta-oxidation continues until a double bond is reached in the fatty acid chain.
- At this point, additional enzymes such as isomerase and reductase are needed to assist in converting the substrates.
- It is important to note that the energy yield from fatty acid metabolism is lower than that from carbohydrates.
- Specifically, each double bond present in a fatty acid reduces the energy yield by 1.5 ATP.
- The metabolism of unsaturated fatty acids requires specific enzymes, and in some cases, both isomerases and reductases are involved in the process.
Clinical Relevance of Fatty Acid Metabolism
- Understanding fatty acid metabolism is crucial for diagnosing metabolic disorders, as disruptions in these pathways can lead to serious health issues.
- Ongoing research is focused on developing therapeutic interventions related to fatty acid metabolism.
- This process is vital for energy production and maintaining metabolic health, playing a critical role in cellular functions and overall health.
Refsum's Disease
Refsum's disease occurs due to the absence of phytanoyl-CoA hydroxylase (also known as Phytanoyl Acid Oxidase. in the peroxisomes.
The characteristic symptoms of classic Refsum's disease include:
- Vision impairment caused by retinitis pigmentosa
- Ichthyosis
- Peripheral neuropathy
- Ataxia
- Night blindness
Classic Refsum disease typically manifests in young adulthood, although symptoms such as visual disturbances, night blindness, ichthyosis, and peripheral neuropathy can begin in childhood.
It is advisable to reduce dairy product consumption and increase the intake of green leafy vegetables.
Ketone Bodies
- Ketone bodies are produced during extended periods of fasting or when carbohydrate intake is low.
- This process involves the hydroxylation of the last methyl group, resulting in short-chain dicarboxylic acids.
Quick Review—Sites of Oxidation of Fatty Acids:
- Beta oxidation of fatty acids occurs in mitochondria and peroxisomes up to octanoyl-CoA, with the remainder of the process taking place in mitochondria.
- Alpha oxidation takes place in peroxisomes and the smooth endoplasmic reticulum.
- Omega oxidation occurs in the smooth endoplasmic reticulum.
Ketogenesis occurs under specific metabolic conditions when there is increased mobilization of fatty acids.
- Secondary ketone bodies include acetone and beta-hydroxybutyrate.
- The blood concentration of ketone bodies does not exceed 5 mM.
- In healthy individuals, the ratio of beta-hydroxybutyrate to acetoacetate is 1:1.
- This ratio increases during ketosis.
Ketone Body Synthesis
- Ketone bodies are synthesized exclusively in the mitochondria of the liver.
- The process begins with acetoacetyl-CoA, which is derived from beta oxidation.
- HMG-CoA synthase plays a crucial role in the synthesis of HMG-CoA from acetoacetyl-CoA and acetyl-CoA.
- This enzyme is essential for both cholesterol synthesis and the production of ketone bodies.
Steps of Ketone Body Synthesis
The synthesis of ketone bodies involves several key steps:
- Two molecules of acetyl-CoA combine to form acetoacetyl-CoA through a reversal of the thiolase reaction.
- Acetoacetyl-CoA then reacts with another acetyl-CoA molecule to produce 3-hydroxy-3-methylglutaryl-CoA (HMG-CoA) through the action of hydroxy-3-methylglutaryl-CoA synthase.
- HMG-CoA lyase catalyzes the breakdown of HMG-CoA into acetoacetate and acetyl-CoA.
- Acetoacetate can spontaneously decarboxylate to form acetone.
- Acetoacetate can also be converted into beta-hydroxybutyrate with the assistance of beta-hydroxybutyrate dehydrogenase.
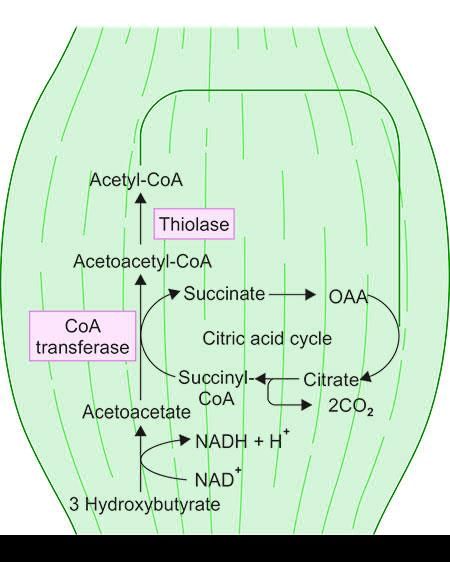 Utilization of ketone bodies
Utilization of ketone bodies
The pathways involving HMG-CoA are significant for:
- Ketone body synthesis
- Cholesterol synthesis
- Utilization of ketone bodies
- Energetics of fatty acid oxidation when ketone bodies are the end products.
Utilizing ketone bodies can serve as an alternative energy source during prolonged fasting or when carbohydrate intake is restricted.
Ketone Bodies as Fuel for Extrahepatic Tissues
- Most organs utilize ketone bodies as an energy source, with the exceptions being the liver and red blood cells (RBCs).
Steps for Utilization of Ketone Bodies
- In extrahepatic tissues, acetoacetate is converted into acetoacetyl-CoA by the enzyme succinyl-CoA-acetoacetate CoA transferase, also known as thiophorase.
- During this process, succinyl-CoA donates CoA, leading to the formation of acetoacetyl-CoA.
- Subsequently, acetoacetyl-CoA is cleaved into two molecules of acetyl-CoA with the help of the enzyme thiolase.
Test for Ketone Bodies in Urine
- Ketone bodies can be detected in urine samples using specific tests.
- One of the ketone bodies that can be measured in these tests is acetone.
Quick Review — Ketone Bodies
- Ketone bodies are produced during fasting or carbohydrate restriction and serve as an alternative energy source for various tissues.
- They include acetoacetate, β-hydroxybutyrate, and acetone.
- Understanding ketone bodies is important for metabolic health.
- Starvation
- Brain
- Heart
- Amino Acid
- Fatty Acid/Ketone bodies
Clinical Connection: Fatty Liver
Lipids, primarily as
triacylglycerol, can accumulate in the liver, leading to conditions like Nonalcoholic Fatty Liver Disease (NAFLD), which is the most common liver disorder.
Stages of Progression of NAFLD
- Nonalcoholic Steatohepatitis (NASH)is a severe form of NAFLD that can progress to serious liver conditions such as:
- Cirrhosis
- Hepatocarcinoma (liver cancer)
- Liver failure
- The production and release of triacylglycerol in the liver are influenced by various metabolic factors.
- When plasma free fatty acid levels increase, the liver's ability to produce Very Low-Density Lipoprotein (VLDL) is overwhelmed. This imbalance leads to the accumulation of triacylglycerol in the liver, contributing to the development of fatty liver.
Reasons for the Condition
- Starvation
- High-Fat Diets
- Uncontrolled Diabetes Mellitus
- Twin Lamb Disease
This condition arises from a metabolic block in the production of plasma lipoproteins, leading to the accumulation of triacylglycerol.
Possible Causes
- Block in Apolipoprotein Synthesis: This can occur in conditions like Kwashiorkor.
- Block in Lipoprotein Synthesis: This involves the synthesis of lipoproteins from lipids and apolipoproteins.
- Phospholipid Provision Failure: There may be a failure in providing phospholipids, which are essential components of lipoproteins.
- Secretory Mechanism Failure: The secretory mechanism itself may fail, leading to this condition.
- Orotic Acid: The presence of orotic acid may contribute to this condition.
- Antibiotic Puromycin: Puromycin inhibits protein synthesis and, along with substances like ethionine, carbon tetrachloride, chloroform, phosphorus, lead, and related compounds, can contribute to this condition.
Lipotropic Factors
- Choline and Betaine: These are essential nutrients that play a crucial role in fat metabolism and liver function. Choline is a precursor to phosphatidylcholine, a vital component of cell membranes and lipoproteins. Betaine, on the other hand, helps in the methylation process and supports liver health.
- Vitamin E: This antioxidant vitamin helps protect liver cells from oxidative damage. It also plays a role in preventing the accumulation of fat in the liver by promoting the breakdown of fatty acids.
- Methionine and S-Adenosylmethionine (SAMe): Methionine is an essential amino acid that contributes to the synthesis of proteins and other important molecules in the body. SAMe, a derivative of methionine, is involved in various methylation reactions and helps in the detoxification processes in the liver.
- Selenium: This trace mineral is important for the activity of antioxidant enzymes in the liver, such as glutathione peroxidase. It helps in reducing oxidative stress and supports overall liver function.
- Pyridoxine (Vitamin B6) and Pantothenic Acid (Vitamin B5): These B vitamins are involved in various metabolic processes, including the metabolism of fats, proteins, and carbohydrates. They support the overall metabolic functions of the liver and help in maintaining a healthy balance of nutrients.
Alcoholic Fatty Liver
Alcoholic liver disease (ALD) is a consequence of excessive alcohol consumption and has the potential to progress to cirrhosis, a severe liver condition. The accumulation of fat in the liver occurs due to several interconnected factors:
- Reduced Fatty Acid Breakdown and Increased Fat Production: In ALD, there is a decrease in the breakdown of fatty acids, which contributes to the accumulation of fat in the liver. Simultaneously, there is an increase in the production of fat, exacerbating the condition.
- Ethanol Metabolism: The metabolism of ethanol by enzymes such as alcohol dehydrogenase and aldehyde dehydrogenase leads to an excess production of certain metabolites. This process is associated with an increased ratio of NADH to NAD, which disrupts normal metabolic processes.
- Triglyceride Formation: The altered metabolic state in ALD promotes the increased formation of triglycerides from fatty acids, further contributing to fat accumulation in the liver.
- Hyperlactic Acidosis: There is an elevation in the levels of lactate relative to pyruvate, leading to hyperlactic acidosis. This condition affects the excretion of uric acid and can worsen gout, a related health issue.
Cholesterol
Cholesterol is primarily found in animals and is not significantly present in plants. It is composed of a steroid base known as ‘Cyclopentanoperhydrophenanthrene.’
Cholesterol can be produced by all tissues with nucleated cells, with significant production occurring in the:
- Liver
- Adrenal cortex
- Testes
- Ovaries
- Intestinal organelles, especially in the smooth endoplasmic reticulum and cytoplasm
Major Sites: The main locations for cholesterol production include:
- Liver
- Adrenal cortex
- Testes
- Ovaries
- Intestinal organelles
Steps of Cholesterol Synthesis
Formation of HMG CoA
- Two molecules of Acetyl CoA (2C) combine to create Acetoacetyl CoA.
- Acetoacetyl CoA then joins with another molecule of Acetyl CoA to form HMG CoA with the help of the enzyme HMG CoA synthase.
Synthesis of Mevalonate
- HMG CoA (6C) is transformed into Mevalonate (6C) by the enzyme HMG CoA Reductase.
- This step is the rate-limiting step in the production of cholesterol.
- Statins act as competitive inhibitors of HMG CoA Reductase.
Generation of Isoprenoid Units (5C)
- Mevalonate undergoes decarboxylation and phosphorylation.
Condensation of 5 Carbon isoprenoid units to form Squalene (30C)
- Two 5C units combine to create a 10C compound – Squalene.
- Squalene is then transformed into 2,3-oxidosqualene, which is converted into lanosterol, an important precursor in the production of sterols.
- Lanosterol is subsequently changed into cholesterol through several enzymatic reactions.
Formation of Cholesterol
A straight-chain 30C molecule folds to create a structure that eventually leads to cholesterol.
The intermediates in the conversion of Lanosterol to Cholesterol include:
- 14 Desmethyl Lanosterol
- Zymosterol
- Desmosterol
- First Cyclical Compound formed
- First Steroid Compound formed
Compare–Cholesterol Synthesis and Ketone Body Synthesis
Cholesterol synthesis:
- Cytoplasm/Smooth Endoplasmic Reticulum
- HMG CoA as an intermediate
- Yes
- Yes, the regulatory step
- Yes, the rate limiting step
- HMG CoA Reductase
Remember:
- Cytoplasmic HMG CoA Synthase is for cholesterol synthesis
- Mitochondrial HMG CoA Synthase is for ketone body synthesis
Uses of Isoprenoid units in Farnesyl and Geranyl Diphosphate
- Farnesyl and Geranyl Diphosphate are precursors for the synthesis of Dolichol and Ubiquinone.
Prenylation is the process of attaching isoprenoid units to proteins.
- It helps in the localisation of certain proteins in membranes.
- It is crucial for the function of G proteins.
- It is involved in synthesising molecules that are part of cellular signalling.
- It is significant for post-translational modification of proteins.
Regulation of Cholesterol Synthesis
- Feedback Inhibition: HMG CoA Reductase is inhibited by feedback mechanisms.
- Feedback Regulation: Cholesterol decreases the production of HMG CoA Reductase. This regulation occurs through the Sterol Regulatory Element Binding Protein (SREBP).
- Hormonal Regulation: HMG CoA Reductase is more active when it is dephosphorylated.
- Insulin and Thyroxine enhance the activity of HMG CoA Reductase.
- Glucagon and Glucocorticoids decrease the activity of HMG CoA Reductase.
Mechanism of Regulation by Insulin
- Insulin plays a crucial role in regulating glucose levels and fat production within the body.
- It facilitates the uptake of glucose by cells, leading to a decrease in blood sugar levels.
- Additionally, insulin is involved in the synthesis of fatty acids and cholesterol, both of which are essential for various cellular functions.
- A key aspect of insulin's regulatory function is its control over the enzyme HMG CoA reductase, which is pivotal in the biosynthesis of cholesterol.
- Insulin acts antagonistically to glucagon, a hormone that raises blood sugar levels, thereby helping to maintain energy balance within the body.
- Furthermore, insulin promotes the conversion of glucose into fatty acids and inhibits the breakdown of fats, processes that are vital for energy homeostasis.
- Consequently, HMG CoA reductase is more active when insulin levels are elevated.
By Two Mechanisms
First Method
- Insulin directly activates HMG CoA reductase, promoting its active form.
- It can also inhibit this enzyme by adding a phosphate group to it, a process known as phosphorylation.
- The regulation of cholesterol production is crucial for maintaining proper cell function and energy balance within the body.
- Insulin's involvement in fat metabolism is significant for how the body stores and utilizes energy.
Key Points Regarding Cholesterol Regulation
- Cholesterol is primarily synthesized in the liver and is essential for various physiological functions, including maintaining cell membrane integrity and producing hormones.
- Although cholesterol cannot be used directly as an energy source, it is a fundamental component of cell membranes and serves as a precursor for steroid hormones.
- Cholesterol can be converted into bile acids, which are crucial for the digestion and absorption of dietary fats.
- Additionally, cholesterol is a precursor for Vitamin D and sex hormones such as oestrogen and testosterone.
- Specialized products derived from cholesterol include:
- Bile acids (which represent the excretory form of cholesterol)
- Vitamin D
- Sex hormones
- Corticosteroids
|
110 videos|130 docs|117 tests
|
FAQs on Metabolism of Lipids - 1 Chapter Notes - Chemistry for ACT
| 1. What are the main enzymes involved in the digestion of lipids? |  |
| 2. How are lipids absorbed in the body? |  |
| 3. What is the process of triacylglycerol synthesis? |  |
| 4. What factors activate hormone-sensitive lipase (HSL)? |  |
| 5. What mechanisms lead to the inactivation of hormone-sensitive lipase? |  |














