Metabolism of Lipids - 2 Chapter Notes | Chemistry for ACT PDF Download
| Table of contents |

|
| Bile Acid Synthesis |

|
| Metabolism of Lipoproteins |

|
| Disorders of Lipoprotein Metabolism (Dyslipoproteinemias) |

|
| Pharmacologic Treatment of Lipoprotein Disorders |

|
Bile Acid Synthesis
Bile Acids and Their Site of Synthesis
Primary Bile Acidsare produced in the liver and are the most common bile acids in mammals. These include:
- Cholic Acid
- Chenodeoxycholic Acid, also known as Chenic acid
Secondary Bile Acidsare formed in the intestine and include:
- Deoxycholic Acid
- Lithocholic Acid
Steps of Bile Acid Synthesis
- The synthesis of primary bile acids begins in the liver.
- The process typically involves monooxygenases, which require NADPH and molecular oxygen to function effectively.
- Subsequent steps lead to the formation of Cholic acid through various subpathways involving different enzymes and intermediates.
- In humans, there is a specific ratio of Glycine to Taurinein bile acid synthesis:
- When combined with glycine, it forms Glycocholic Acid and Glycochenodeoxycholic Acid
- When combined with taurine, it forms Taurocholic Acid
- Bile Salts, which are essential for digestion, include sodium and potassium salts.
- In the intestine, secondary bile acidsare synthesized by bacterial enzymes that modify primary bile acids:
- Cholic acid is converted to Deoxycholic acid
- Chenodeoxycholic acid is converted to Lithocholic acid
- Enterohepatic Circulation refers to the process where 98–99% of absorbed bile acids are recycled back to the liver via portal circulation.
- Lithocholic acid is further modified in the body.
Regulation of Bile Acid Synthesis
- The synthesis of bile acids is primarily regulated at the step involving cholesterol 7α-hydroxylase (CYP7A1), which is a key enzyme in the process.
- The activity of this enzyme is influenced by feedback from the farnesoid X receptor (FXR), a nuclear bile acid-binding receptor.
- When the levels of bile acids in the enterohepatic circulation are low, the body responds by increasing the production of bile acids to restore the supply.
- Chenodeoxycholic acid is an important component in this regulatory mechanism.
- The regulation of bile acid synthesis involves intricate interactions among various nuclear receptors and signaling pathways, which are crucial for maintaining the balance of cholesterol and bile acid levels in the body.
Overview of Lipoproteins
Lipoproteins are complex lipids consisting of both lipids and proteins. They play a crucial role in the transport of lipids, such as cholesterol and triglycerides, throughout the body.
Lipoproteins are classified based on their density and function. The main types include:
- Chylomicrons
- Very low-density lipoproteins (VLDL)
- Low-density lipoproteins (LDL)
- High-density lipoproteins (HDL)
These lipoprotein particles have a core of hydrophobic lipids surrounded by a shell of proteins and phospholipids. This unique structure allows them to interact with both lipids and water in the bloodstream, facilitating the transport of essential lipids throughout the body.
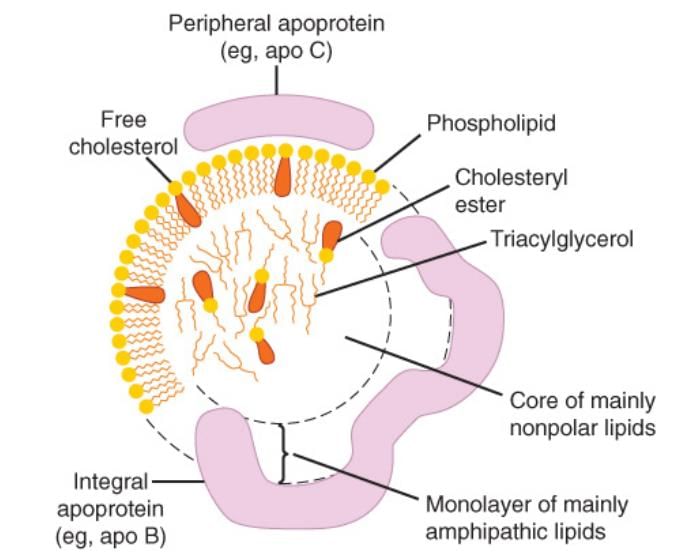
Structure of Lipoproteins
- Lipoproteins consist of a nonpolar core surrounded by a single layer of amphipathic lipids.
- The nonpolar lipid core primarily contains triacylglycerol and cholesteryl ester.
- Surrounding this core is a single layer of phospholipids and proteins, with the polar groups of these molecules facing outward, allowing interaction with water. This structure is similar to that of a cell membrane.
Major Classes of Lipoproteins
The major classes of lipoproteins, which play essential roles in lipid transport and metabolism, include:
- Chylomicrons: These are the largest lipoproteins and are primarily responsible for transporting dietary lipids from the intestines to other parts of the body.
- Very Low-Density Lipoproteins (VLDL): VLDL is produced by the liver and is involved in transporting endogenous lipids, mainly triglycerides, to peripheral tissues.
- Low-Density Lipoproteins (LDL): LDL is derived from VLDL and is responsible for delivering cholesterol to cells throughout the body. It is often referred to as "bad cholesterol" due to its association with atherosclerosis.
- High-Density Lipoproteins (HDL): HDL is involved in reverse cholesterol transport, picking up excess cholesterol from peripheral tissues and transporting it back to the liver for excretion. It is known as "good cholesterol" because higher levels of HDL are associated with a lower risk of cardiovascular disease.
Lipoproteins
- Chylomicrons (Least Density)
- Very Low Density Lipoproteins (VLDL)
- Low Density Lipoproteins (LDL)
- Intermediate Density Lipoproteins (IDL)
- High Density Lipoproteins (HDL)
 Structure of lipoprotein
Structure of lipoprotein
Based on Electrophoretic Separation
- Chylomicrons
- Very Low Density Lipoproteins
- Low Density Lipoproteins
- Intermediate Density Lipoproteins
Effect of Protein Content on Mobility
- The higher the protein content, the faster the mobility of lipoproteins.
- Chylomicrons, which have the least protein content, remain at the origin, while HDL, with the highest protein content, migrates the farthest.
- An exception is that VLDL and IDL, despite having less protein than HDL, still contain notable protein levels.
Lipoproteins and Their Functions
- Carry dietary Triacylglycerol (Exogenous TAGs)
- Carry endogenous Triacylglycerol
- Deliver Cholesterol and cholesterol ester to tissues
- Deliver cholesterol from peripheral tissues to the liver
- The protein part of lipoprotein is apolipoprotein.
- The apolipoproteins can be removed and transferred to other lipoproteins or peripheral proteins.
- The major apolipoprotein in LDL and VLDL is apo B.
- Chylomicrons contain a truncated apolipoprotein.
Apolipoproteins and Their Functions
- Apolipoproteins play a crucial role in lipid metabolism and transport within the body.
- They are involved in various processes such as activating enzymes, inhibiting certain lipases, and acting as ligands for receptors.
Functions of Apolipoproteins:
- Activates Lecithin Cholesterol Acyl Transferase (LCAT): LCAT is an enzyme that plays a vital role in the metabolism of cholesterol esters. Apolipoproteins help activate this enzyme, facilitating the conversion of free cholesterol into cholesterol esters, which are then transported in lipoproteins.
- Inhibits Lipoprotein Lipase: Some apolipoproteins inhibit lipoprotein lipase, an enzyme responsible for the breakdown of triglycerides in lipoproteins. This inhibition can regulate the levels of circulating lipoproteins and their lipid content.
- Promotes lipoprotein lipase-mediated triacylglycerol metabolism. Other apolipoproteins promote the activity of lipoprotein lipase, enhancing the breakdown of triglycerides in lipoproteins and facilitating the release of fatty acids for energy or storage.
- Acts as a ligand for the LDL receptor: Apolipoproteins serve as ligands for the low-density lipoprotein (LDL) receptor, facilitating the uptake of chylomicron remnants and very low-density lipoprotein (VLDL) remnants, also known as intermediate-density lipoproteins (IDL). This process is crucial for regulating lipid levels in the body.
- Inhibits Cholesterol Ester Transfer Protein (CETP): Some apolipoproteins inhibit CETP, an enzyme that facilitates the transfer of cholesterol esters between lipoproteins. This inhibition can influence the distribution of cholesterol esters among different lipoproteins.
- Activates lipoprotein lipase: Certain apolipoproteins activate lipoprotein lipase, promoting the breakdown of triglycerides in lipoproteins and the release of fatty acids for various metabolic processes.
More about Apolipoproteins
- Apolipoproteins are proteins that play a crucial role in the metabolism and transport of lipids in the body. They are integral components of lipoproteins, which are responsible for carrying fats and cholesterol through the bloodstream.
- There are various isoforms of apolipoproteins, including Apo E, each with specific functions and roles in lipid metabolism. Apo E isoforms, in particular, are significant in regulating the levels of triglycerides and cholesterol in the body.
- Different isoforms of Apo E can have varying effects on lipid metabolism. For instance, some isoforms may enhance the clearance of lipoproteins from the bloodstream, thereby reducing lipid levels.
- Conversely, certain Apo E isoforms may be linked to conditions like hyperlipoproteinemia, where there is an abnormal increase in lipoprotein levels in the blood. One such condition associated with specific Apo E isoforms is Familial Dysbeta Lipoproteinemia, a genetic disorder characterized by elevated levels of triglycerides and cholesterol in the blood due to impaired lipoprotein clearance.
Lipoproteins at a Glance
- Carry exogenous (dietary) triacylglycerol from the intestine to tissues.
- Carry dietary cholesterol and cholesterol ester into cells.
- Carry endogenous TAG from the liver to peripheral tissues.
- Also known as VLDL remnant.
- Broad Beta Lipoprotein.
- Least Electrophoretic Mobility [Remains at the point of origin during electrophoresis].
- The degradation of LDL in extrahepatic tissue is responsible for the deposition of cholesterol and other lipids.
- Alpha lipoprotein has the least diameter and maximum electrophoretic mobility.
- It carries cholesterol from peripheral tissues to the liver and other steroidogenic tissues.
- This process is called Reverse Cholesterol Transport.
- This makes HDL cholesterol 'the good cholesterol'.
- The major role of HDL is to act as a repository for apo C and apo E required for the metabolism of lipoproteins.
Metabolism of Lipoproteins
Chylomicrons are large lipoprotein particles that play a crucial role in the metabolism of dietary fats. They are formed in the intestine and are responsible for transporting triglycerides (TAG) from the gut to various tissues in the body. The process of chylomicron metabolism involves several key steps:
Step 1: Formation of Nascent Chylomicron
- The process of creating nascent chylomicrons occurs in the intestine.
Step 2: Formation of Mature Chylomicron
- Chylomicrons undergo maturation in the lining of the intestine.
Step 3: Formation of Remnant Chylomicron
- Apo C-II plays a crucial role in activating Lipoprotein Lipase.
- Lipoprotein Lipase is an enzyme located on the walls of blood capillaries. It is attached to the endothelium by negatively charged proteoglycan chains of Heparan sulfate.
- This enzyme is responsible for breaking down TAG in mature chylomicrons into fatty acids and glycerol, leading to the formation of chylomicron remnants.
- The remnants produced after the breakdown are subsequently absorbed by the liver.
Step IV - Uptake of Remnant Chylomicron
- The liver takes up the chylomicron remnant through its receptors.
- This uptake is facilitated by apo E using two types of apo E dependent receptors: the LDL receptor and LDL receptor related proteins.
- Hepatic Lipase breaks down the triacylglycerol in the remnants.
VLDL Metabolism
Step I - Formation of Nascent VLDL
- The liver assembles nascent VLDL which carries lipids.
Step II: Transformation into Mature VLDL
- Nascent VLDL undergoes maturation to become mature VLDL with the help of various enzymes.
Step III: Formation of Remnant VLDL (IDL)
- Apo C-II plays a crucial role in activating lipoprotein lipase.
- Lipoprotein lipase, located in the walls of capillaries in extrahepatic tissues, is essential for this process.
- The remnant VLDL, also known as IDL, is taken up by the liver through apolipoproteins, which facilitate this uptake.
Step IV: Fate of IDL
- VLDL remnant, identified as IDL, is taken up by the liver with the assistance of apo E.
- Apo E plays a vital role in helping hepatocytes, the liver cells, clear IDL from the circulation.
Step V: Uptake of LDL by Tissues
- LDL is internalized by the LDL receptor through receptor-mediated endocytosis.
- Apo B100 is crucial for the binding between LDL and its receptor.
- Approximately 30% of LDL is degraded in the liver, where it undergoes further processing or repackaging.
- LDL receptors are present in both hepatic and extrahepatic tissues.
- These receptors are located on the cell surface, with pits coated on the cytosolic side with clathrin.
- LDL is taken up in its entirety through receptor-mediated endocytosis, a process where cells internalize substances by forming vesicles.
- High levels of LDL lead to a decrease in the synthesis of LDL receptors, resulting in reduced LDL uptake.
- Increased levels of polyunsaturated fatty acids (PUFA) and monounsaturated fatty acids (MUFA) are associated with the upregulation of LDL receptors, enhancing the breakdown rate of LDL.
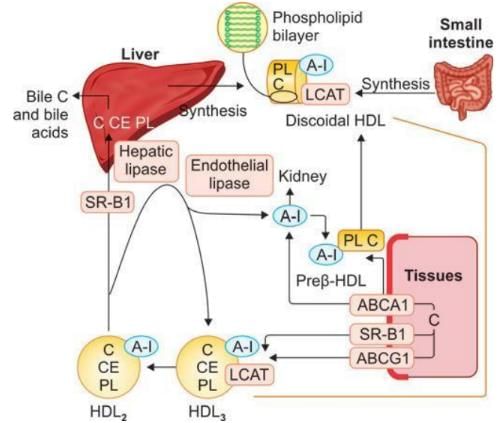
HDL Metabolism and Reverse Cholesterol Transport
- Nascent HDL is generated and secreted by the liver.
- Initially, HDL is in a disk shape, composed of a phospholipid bilayer and cholesterol.
- Lecithin Cholesterol Acyl Transferase (LCAT) binds to cholesterol on HDL.
- LCAT converts cholesterol into nonpolar cholesterol esters.
- This conversion involves using cholesterol and lecithin to produce cholesterol esters and lysolecithin.
- As a result of this process, HDL becomes less dense and transforms into a spherical shape with a core of cholesterol esters.
- HDL scavenges cholesterol from tissues via Class B scavenger receptors, facilitating the transport of cholesterol from peripheral tissues back to the liver.
- LCAT continues its action on cholesterol in HDL3, further converting it into cholesterol esters.
- Consequently, HDL transports cholesterol and cholesterol esters to the liver through the SR-B1 receptor, directs them to steroid-producing tissues, or undergoes processing by hepatic lipase.
- HDL plays a crucial role in cholesterol metabolism, including the transfer of cholesterol to steroid-producing tissues, which is essential for the synthesis of steroid hormones.
- Enzymes such as LCAT and hepatic lipase are pivotal in the metabolism of HDL.
 Metabolism of HDL
Metabolism of HDL
Enzymes Involved in Reverse Cholesterol Transport
- LCAT is the primary enzyme responsible for the majority of plasma cholesteryl esters.
- Cholesterol esters, being nonpolar, move into the interior of HDL particles.
- This movement creates a concentration gradient that facilitates the influx of cholesterol into HDL.
- As a result, HDL is able to carry out reverse cholesterol transport effectively.
- HDL exchanges cholesterol esters with other lipoproteins such as VLDL, IDL, and LDL.
- This exchange process helps to reduce product inhibition caused by cholesteryl esters.
- LCAT also plays a crucial role in converting cholesterol into cholesterol esters within HDL particles.
Receptors Involved in Reverse Cholesterol Transport
Class B Scavenger Receptor B1 (SR-B1)
- In the liver and steroid-producing tissues, SR-B1 plays a crucial role in delivering cholesterol and cholesterol esters to the tissues.
- In the liver, excess cholesterol is converted into bile acids for excretion.
- In steroidogenic tissues, cholesterol is utilized for the synthesis of steroid hormones.
- SR-B1 also facilitates the uptake of cholesterol esters from peripheral organs into the tissues.
Cholesterol Homeostasis
- The liver is responsible for maintaining cholesterol homeostasis by regulating its uptake, synthesis, and excretion.
- ABCA1 is primarily involved in transporting cholesterol to nascent HDL particles.
HDL Fractions
- Nascent HDL or Discoidal HDL. This fraction consists of a phospholipid bilayer, cholesterol, and Apo A-I.
- Spherical HDL or HDL3. Contains cholesterol, cholesteryl ester, phospholipid, Apo A-I, and LCAT (Lecithin-Cholesterol Acyltransferase).
- Spherical less dense HDL or HDL2. Composed of cholesterol, cholesteryl ester, phospholipid, and Apo A-I.
- Prebeta HDL. This fraction is highly effective in transporting cholesterol from tissues; it contains Apo A-I, cholesterol, and phospholipid.
- ABCA1 primarily transfers cholesterol to the Prebeta HDL Cycle.
- The process of exchanging HDL2 and HDL3 is known as HDL remodeling.
Transport of Cholesterol between Tissues
- Cholesterol is transported to the liver with the assistance of chylomicrons.
- Approximately 95% of chylomicron cholesterol is delivered to the liver.
- The liver regulates the distribution of cholesterol to other tissues.
- A significant portion of cholesterol released in Very Low-Density Lipoprotein (VLDL) remains in the liver.
- Low-Density Lipoprotein (LDL) cholesterol is taken up by the liver and other peripheral tissues.
Cholesterol Balance in the Tissues
The Indian economy has shown resilience in the face of global challenges, with the World Bank projecting a growth rate of 6.3% for the fiscal year 2023-24. This growth is supported by robust domestic demand, driven by increased rural consumption and public sector investment. Despite global uncertainties, including geopolitical tensions and inflationary pressures, India remains a bright spot in the global economy, benefiting from strong agricultural performance and a rebound in services and manufacturing sectors.
Current Economic Scenario
Growth Drivers
- Domestic Demand: The primary driver of India’s economic growth is domestic demand, which remains strong due to various factors.
- Rural Consumption: There has been a significant increase in rural consumption, aided by factors such as good monsoon rains, which have boosted agricultural output and rural incomes.
- Public Sector Investment: The government’s focus on infrastructure and development projects has led to increased public sector investment, further stimulating economic activity.
Resilience Amid Global Challenges
- Geopolitical Tensions: India has managed to navigate through global uncertainties, including geopolitical tensions that have affected trade and economic stability in various regions.
- Inflationary Pressures: Despite facing inflationary pressures, particularly in food and energy prices, India’s economy has shown the ability to adapt and maintain growth.
Sectoral Performance
- Agriculture: The agricultural sector has performed strongly, contributing to overall economic stability and growth. The good monsoon and increased agricultural productivity have played a crucial role.
- Services and Manufacturing: There has been a rebound in the services and manufacturing sectors, which are vital for sustaining economic growth. The revival in these sectors has been supported by domestic demand and global recovery trends.
Outlook
Looking ahead, the Indian economy is expected to maintain a strong growth trajectory, supported by ongoing domestic demand, government initiatives, and sectoral recovery. The World Bank’s positive outlook reflects confidence in India’s economic fundamentals and the ability to navigate global challenges effectively.
Disorders of Lipoprotein Metabolism (Dyslipoproteinemias)
Hyperlipoproteinemias
- Hyperlipoproteinemias are classified according to the specific types of lipoprotein particles present in the blood.
- Here’s a detailed overview of various types of Hyperlipoproteinemias:
Frederickson Type
Type I: Familial Chylomicronemia Syndrome (FCS)
- Molecular Defect: Caused by mutations in the genes responsible for lipoprotein lipase (LPL) or apolipoprotein C-II (Apo C-II). These proteins are crucial for the breakdown of triglycerides in the bloodstream.
- Genetic Transmission: Inherited in an autosomal recessive manner, meaning both copies of the mutated gene (one from each parent) are necessary for the disease to manifest.
- Estimated Incidence: Approximately 1 in 1,000,000 individuals, making it a very rare condition.
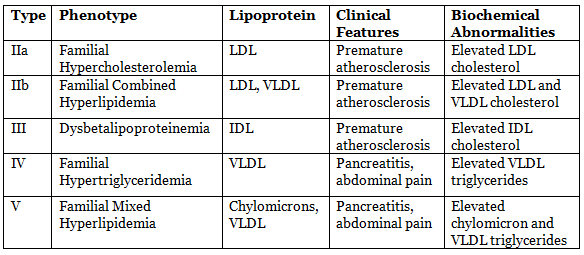
Type IIa: Familial Hypercholesterolemia (FH)
- Molecular Defect: Caused by mutations that affect the LDL receptor, which is responsible for clearing low-density lipoprotein (LDL) cholesterol from the bloodstream.
- Genetic Transmission: Typically inherited in an autosomal dominant manner, meaning only one copy of the mutated gene (from either parent) is sufficient to cause the disorder.
- Estimated Incidence: About 1 in 500 individuals, making it one of the most common genetic disorders.
Familial Defective Apo B (FDB)
- Relation: This condition is related to Autosomal Dominant Hypercholesterolemia Type II (ADH Type II).
- Estimated Incidence: Approximately 1 in 1,000,000 individuals, indicating its rarity.
Autosomal Dominant Hypercholesterolemia Type III (ADH Type III)
- Molecular Defect: Involves mutations in the PCSK9 gene, which plays a role in regulating LDL receptors.
- Estimated Incidence: About 1 in 10,000 individuals, indicating a relatively higher frequency compared to other types.
Autosomal Recessive Hypercholesterolemia (ARH)
- Molecular Defect: Caused by mutations in the LDL receptor adapter protein (LDLRAP) gene.
- Genetic Transmission: Inherited in an autosomal recessive manner.
- Related Conditions: Often associated with sitosterolemia, a condition characterized by the accumulation of plant sterols in the body.
Type IIb: Familial Combined Hyperlipidemia (FCHL)
- Hyperlipoproteinemia Type IIb is characterized by an increase in both cholesterol and triglycerides, leading to a mixed hyperlipidemic state.
- The condition is often associated with insulin resistance, obesity, and increased cardiovascular risk.
- Genetic factors play a significant role in the development of FCHL, and it is commonly observed in families with a history of premature cardiovascular disease.
- Management typically involves lifestyle modifications and pharmacotherapy aimed at lowering lipid levels and reducing cardiovascular risk.
Type IIb: Familial Dysbetalipoproteinemia (FDBL)
- Familial Dysbetalipoproteinemia (FDBL) is a genetic disorder characterized by the impaired clearance of cholesterol-rich lipoproteins from the bloodstream.
- The condition is associated with mutations in the apolipoprotein E (ApoE) gene, leading to the accumulation of IDL and chylomicron remnants.
- Clinically, FDBL presents with premature atherosclerosis, xanthomas, and elevated cholesterol levels.
- Management involves dietary modifications and lipid-lowering therapies to reduce cholesterol levels and cardiovascular risk.
Type IV: Familial Hypertriglyceridemia (FHTG)
- Characteristics: FHTG is characterized by elevated levels of VLDL in the bloodstream, leading to hypertriglyceridemia.
- Clinical Features: Patients may present with abdominal pain and pancreatitis due to the high triglyceride levels.
- Genetic Transmission: The condition is often inherited in a familial pattern, with genetic factors contributing to the dysregulation of triglyceride metabolism.
- Incidence: FHTG is estimated to occur in 1 in 100,000 individuals, although the exact prevalence may vary.
Type V
- Phenotype:IIb
- Lipoprotein: Elevated levels of chylomicrons and VLDL.
- Clinical Features: Patients may experience symptoms such as abdominal pain and pancreatitis due to high triglyceride levels.
- Genetic Transmission: Inherited in a familial pattern, often associated with genetic predisposition.
- Estimated Incidence: Occurs in approximately 1 in 100,000 individuals, although prevalence may vary.
Primary Hyperlipoproteinemias Leading to Increased Triglycerides
Familial Chylomicronemia Syndrome (Type I Hyperlipoproteinemia)
Biochemical Abnormalities
- The lipoproteins that accumulate in this condition are chylomicrons and VLDL (Very Low-Density Lipoprotein).
- Lipoprotein Lipase is crucial for the breakdown of triglycerides, which are predominantly found in chylomicrons and VLDL.
Clinical Presentation
- Patients may present with symptoms of acute pancreatitis due to the high levels of triglycerides.
- During a fundoscopic examination, opalescent retinal blood vessels (a condition known as lipemia retinalis) may be observed.
- Lactescent plasma, which appears cloudy, is a common finding in these patients.
- Eruptive xanthomas are characteristic of this condition; these appear as small yellowish-white bumps on the backs, buttocks, and extensor surfaces of the body and can become itchy.
- Hepatosplenomegaly, or enlargement of the liver and spleen, is frequently observed in affected individuals.
Measuring Triglyceride Breakdown Activity in Blood Plasma After Heparin Injection
Observation
- When normal plasma, which contains triglyceride lipolytic activity, is added to post-heparin plasma, it enhances the visibility of lipolytic activity.
Molecular Sequencing of Genes
- There have been recent advancements in the treatment of Familial Chylomicronemia Syndrome.
Gene Therapy: Alipogene Tiparvovec
- This therapy aims to re-encode the function of the LPL variant, promoting the development of skeletal muscle cells.
- Further research is needed to explore the implications and effectiveness of this therapy.
Familial Hypertriglyceridemia (FHTG) (Type IV and V Hyperlipoproteinemia)
- Apo A-V plays a crucial role in linking VLDL with other lipoproteins.
- When there is a loss-of-function mutation in Apo A-V, it leads to increased levels of triglycerides in the bloodstream.
Primary Hyperlipoproteinemias Leading to Hypercholesterolemia
- People with primary hyperlipoproteinemias may have high cholesterol levels due to genetic factors.
- Genetic mutations that impact lipid metabolism can result in abnormal lipid profiles.
- The underlying causes of these disorders involve problems in lipid transport and processing.
- Grasping the genetic foundation of these conditions is essential for developing effective treatments.
Introduction to Autosomal Dominant Hypercholesterolemia Type I (ADH Type I)
- Familial Hypercholesterolemia can be either homozygous or receptor negative.
- Individuals with this condition may present with extremely high levels of LDL-Cholesterol, typically ranging from 400 to 1000 mg/dL.
- Corneal arcus is a common feature, while pancreatitis is not observed.
- Tendon xanthomas are particularly noted on the dorsum of the hands and the Achilles tendon.
- Diagnostic tests such as LDL receptor assay and LDL receptor gene sequencing are used to confirm the condition.
Recent Advances in the Treatment of Homozygous Familial Hypercholesterolemia
- Lomitapide. A medication that inhibits microsomal triglyceride transfer protein (MTP) to reduce the production of atherogenic lipoproteins.
- Mipomersen. An antisense oligonucleotide that decreases LDL cholesterol levels by inhibiting the synthesis of apolipoprotein B.
Familial Defective apo B100 (FDB)
Autosomal Dominant Hypercholesterolemia Type II
- This condition is characterized by elevated cholesterol levels resulting from a defect in apolipoprotein B100.
- Individuals with FDB typically exhibit normal levels of Intermediate Density Lipoprotein (IDL) .
- This is because IDL clearance is not compromised in this condition, distinguishing it from other forms of hyperlipidemia.
Autosomal Dominant Hypercholesterolemia Type III (ADH Type III)
- In ADH Type III, the LDL receptor is sent to the lysosome for breakdown, leading to a faster degradation of LDL.
- This results in the receptor's inability to effectively uptake LDL, causing higher levels of sterols in the body, which triggers various metabolic issues.
Autosomal Recessive Hypercholesterolemia (ARH) Biochemical Abnormalities
- Mutations in the protein responsible for aiding the LDL receptor result in improper intake of LDL particles.
- The lipoprotein-receptor complex fails to move correctly, leading to increased sterol levels in the body and various metabolic problems.
- The sterol transporter family, ABCG5 and ABCG8, found in enterocytes and hepatocytes, pumps plant sterols like sitosterol and campesterol, along with cholesterol, into the gut.
- This process increases intestinal absorption of sterols and reduces biliary excretion, causing elevated plasma and tissue levels of both plant and animal sterols.
- The rise in hepatic sterol levels leads to a decrease in LDL receptor expression, impairing LDL uptake and significantly raising cholesterol levels.
- Clinical manifestations may include tendon xanthomas, early atherosclerosis, and other lipid-related disorders.
- Anisocytosis, poikilocytosis of erythrocytes, and megathrombocytes may occur due to the incorporation of plant sterols into cell membranes.
- Notable features include episodes of hemolysis and splenomegaly.
- Severe hypercholesterolemia resistant to statin treatment is a significant finding, with additional therapies possibly involving bile acid sequestrants.
Primary Hyperlipoproteinemias Causing Both Hypertriglyceridemia and Hypercholesterolemia
Hyperlipoproteinemia refers to a metabolic disorder characterized by the abnormal presence of various lipoproteins in the blood. This condition leads to the accumulation of lipids and lipoproteins, which can contribute to cardiovascular diseases and other health issues.
Type III Hyperlipoproteinemia
Familial Dysbetalipoproteinemia (FDBL), also known as Familial Broad Beta Disease or Remnant Removal Disease, is an autosomal recessive condition caused by genetic alterations in apolipoprotein E (apoE), particularly the apoE2 variant. These genetic changes impair the ability of apoE to bind to lipoproteins, leading to an accumulation of chylomicron and VLDL remnants in the bloodstream.
ApoE Gene Polymorphism
- The APOE gene exhibits variations in its sequence, resulting in three primary isoforms: apoE3 (the most common), apoE2, and apoE4.
- Chylomicron and VLDL remnants containing apoE2 are cleared from the bloodstream more slowly compared to those with other isoforms.
- Individuals homozygous for the E2 allele (E2/E2 genotype) are frequently observed in affected populations.
- Those with the E2/E2 genotype face an increased risk of cardiovascular diseases due to elevated levels of remnant lipoproteins.
- Affected individuals often exhibit very high levels of remnant lipoprotein in their blood.
- Lipoprotein electrophoresis typically reveals a broad beta band in these individuals, indicating the presence of excess remnant lipoproteins.
Hypolipoproteinemias
Hypolipoproteinemias are a group of disorders characterized by low levels of lipoproteins in the blood. Lipoproteins are essential for transporting fats and cholesterol in the body. These conditions can lead to various health issues due to the impaired transport and absorption of dietary fats and fat-soluble vitamins.
Abetalipoproteinemia
Abetalipoproteinemia is a genetic disorder inherited in an autosomal recessive manner. It results from loss-of-function mutations in the gene responsible for producing apolipoprotein B, a crucial component for the transport of lipoproteins.
- Biochemical Defect: The mutations lead to impaired transport of chylomicrons, VLDL, and LDL, causing fat malabsorption.
- Fat-Soluble Vitamins: Patients exhibit a deficiency in fat-soluble vitamins A, D, E, and K due to the disrupted transport.
- Lipoprotein Production: Chylomicrons, VLDLs, LDLs, and apo B are not produced in this condition.
- Growth Failure: Fat malabsorption may lead to growth failure in affected individuals.
Neurological Symptoms: Patients may experience neurological issues such as:
- Loss of deep tendon reflexes
- Sensory ataxia (lack of coordination)
- Spastic gait (difficulty in walking)
- Acanthocytes: The presence of acanthocytes (abnormal red blood cells) is associated with poor fat absorption and vitamin deficiencies.
- Treatment: Management involves a low-fat, high-caloric diet rich in vitamins and large doses of fat-soluble vitamins.
- Vitamin E Deficiency: Most symptoms are due to vitamin E deficiency. Vitamin E and retinyl esters are normally transported by chylomicrons from the intestine and by VLDL from the liver to tissues.
- Consequences of Deficiency: The absence of apo B-containing lipoproteins leads to a significant deficiency of vitamin E, resulting in mild to moderate neurological symptoms and anemia. Bleeding may also occur due to poor absorption of fat-soluble vitamins.
Tangier Disease
- Inheritance Pattern: Tangier Disease is inherited in an autosomal codominant manner.
- Genetic Mutation: The disease is caused by mutations in the ABCA1 gene. This gene is responsible for producing a transporter that moves unesterified cholesterol out of cells.
- Role of ABCA1: ABCA1 is crucial for the initial formation of high-density lipoprotein (HDL) cholesterol. When this transporter is deficient, HDL formation is compromised.
- Cholesterol Accumulation: Without functional ABCA1, cholesterol begins to accumulate in reticuloendothelial cells. This buildup has the potential to lead to atherosclerotic disease, although the exact relationship is not clearly established.
- HDL-C Levels: Individuals with Tangier Disease have very low levels of HDL cholesterol (HDL-C) in their blood.
- Clinical Features: Tangier Disease is associated with a variety of clinical features, which are distinct symptoms observed in affected individuals.
- Clinical Presentation: The way Tangier Disease manifests clinically includes a range of specific symptoms that characterize the condition.
Norum's Disease
Clinical Features:
- Significantly low levels of high-density lipoprotein cholesterol (HDL-C) in the blood.
- Increased levels of free cholesterol.
- Increased levels of lecithin.
Hemolytic Anemia:
- Very low levels of HDL-C.
- Increased levels of free cholesterol.
- Increased levels of lecithin.
Clinical Presentation:
- Symptoms are similar to those seen in classic LCAT deficiency.
- Does not cause hemolytic anemia.
- Does not progress to end-stage renal disease (ESRD).
- Characteristic Findings:
- Presence of elevated lipoproteins in the blood.
Secondary Causes of Increased VLDL Production
- High Carbohydrate Diet: When there is an excess of dietary carbohydrates, the liver converts these into fatty acids, which are then packaged as VLDL (Very Low-Density Lipoprotein).
- Alcohol Consumption: Alcohol significantly impacts triglyceride levels by increasing their synthesis in the liver. It does this by inhibiting the oxidation of fatty acids and promoting the synthesis and secretion of triglycerides and VLDL.
- Obesity and Insulin Resistance: Increased fat tissue (adipocyte mass) in obesity leads to a higher release of free fatty acids from fat stores. Elevated insulin levels in obesity promote fatty acid synthesis. However, insulin resistance decreases the activity of lipoprotein lipase, an enzyme that helps clear triglycerides, resulting in higher levels of triglycerides and free fatty acids in the bloodstream.
- Nephrotic Syndrome: This condition, characterized by the excessive loss of protein in the urine due to kidney dysfunction, is associated with increased VLDL production and subsequently elevated levels of LDL (Low-Density Lipoprotein) cholesterol.
- Cushing's Syndrome: Whether caused by external administration of glucocorticoids or overproduction by the body, glucocorticoids lead to increased production of VLDL in the liver.
Secondary Causes of Reduced Hepatic Uptake of Lipoproteins
Biochemical Defects:
Hypothyroidism
- Thyroid hormone plays a crucial role in enhancing the uptake of lipoproteins by the liver.
- When there is a deficiency of thyroid hormone, as in hypothyroidism, there is a decrease in hepatic LDL (low-density lipoprotein) uptake.
Chronic Kidney Disease
- Chronic kidney disease is associated with reduced hepatic uptake of lipoproteins.
- This condition leads to decreased triglyceride breakdown and impaired clearance of lipoprotein remnants, contributing to lower hepatic uptake.
Liver Disorders and Secondary Hyperlipoproteinemias
- The liver plays a vital role in the formation and clearance of lipoproteins.
- Various liver disorders, such as hepatitis caused by infections, drugs, or alcohol, can disrupt this process.
- Severe cases of hepatitis and liver failure significantly impair liver function and, consequently, lipoprotein metabolism.
Cholestasis
- Cholestasis is characterized by the accumulation of bile acids in the liver.
- In this condition, cholesterol is released into bile, either directly or after being converted into bile acids.
- As a result of cholestasis, free cholesterol and phospholipids are released into the plasma in the form of a lamellar particle known as LP-X.
Estrogen and Secondary Hyperlipoproteinemia
- Estrogen is known to increase the production of lipoproteins, leading to elevated levels of triglycerides and cholesterol in the plasma.
- This specific pattern of lipoproteins reflects increased concentrations of both triglycerides and cholesterol.
Calculation of Lipid Fractions
- Total cholesterol is calculated by summing HDL (high-density lipoprotein) cholesterol, VLDL (very-low-density lipoprotein) cholesterol, and LDL cholesterol.
- This calculation provides an overall total cholesterol level that includes contributions from all three lipid fractions.
- Evaluating these lipid fractions is essential for assessing cardiovascular risk.
Pharmacologic Treatment of Lipoprotein Disorders
For Severe Hypertriglyceridemia
- Fibrates are medications used to address cholesterol issues by promoting the breakdown of triglycerides.
- The goal of treatment is to reduce the production of apoC-III, a protein that hinders the clearance of lipoprotein remnants from the body.
- Additionally, fibrates work by enhancing lipid metabolism through targeted pathways.
- It is crucial to ensure that all scientific claims are accurate and supported by reliable sources or studies to avoid misleading readers.
Fibrates
- Fibrates are the most effective medications for lowering triglyceride levels.
- They also modestly increase HDL-C levels.
- Fibrates are used to treat various types of hyperlipidemias.
Nicotinic Acid (Niacin)
- Niacin works by reducing the breakdown of fats in fat cells.
For Hypercholesterolemia
- HMG-CoA Reductase Inhibitors, commonly known as statins, block a key enzyme involved in cholesterol production.
- This blockage leads to a decrease in cholesterol levels in the body.
Cholesterol Absorption Inhibitors
- Ezetimibe is a medication that helps reduce cholesterol absorption in the intestines.
Bile Acid Sequestrants (Resins)
- Examples of bile acid sequestrants include Cholestyramine, Colestipol, and Colesevelam.
- These drugs work by binding to bile acids in the gut, promoting their excretion instead of reabsorption.
- This process helps maintain bile acid levels in the body.
- Lower bile acid levels in the liver lead to an increase in LDL receptors, which enhances the clearance of LDL from the bloodstream.
LDL Apheresis
- LDL apheresis is a treatment designed for hypercholesterolemia.
- It involves filtering blood through a system that selectively removes LDL particles.
- This procedure is typically used for patients with severe genetic conditions, such as homozygous Familial Hypercholesterolemia.
ATP III Guidelines for Ideal Levels of LDL, HDL, and Cholesterol
- Biochemical Parameters
- Values in mg/dL
- Risk
- Total Cholesterol
- Optimum
- Near or above Optimum
- Borderline High
- Very High
- Desirable
- Borderline High
Predictors of Coronary Artery Diseases
- The risk associated with total cholesterol levels is classified as follows:
- Total cholesterol levels exceeding 240 mg/dL are viewed as very high.
- hs CRP
- Total Cholesterol/HDL ratio
- Apo B/Apo A ratio
- Non-HDL Cholesterols
- The significance of these parameters in evaluating cardiovascular health is vital, as they are key indicators of possible heart disease risk.
|
110 videos|130 docs|117 tests
|
FAQs on Metabolism of Lipids - 2 Chapter Notes - Chemistry for ACT
| 1. What are bile acids and how are they synthesized in the body? |  |
| 2. What is the role of lipoproteins in lipid metabolism? |  |
| 3. What are the common disorders of lipoprotein metabolism? |  |
| 4. What pharmacologic treatments are available for dyslipoproteinemias? |  |
| 5. How can lifestyle changes complement the pharmacologic treatment of lipoprotein disorders? |  |













