Mycobacteria Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
| Introduction |

|
| Mycobacterium tuberculosis Complex |

|
| Non-Tuberculous Mycobacteria |

|
| Mycobacterium leprae |

|
Introduction
Mycobacteria are bacteria that require oxygen to survive. They are classified as weakly gram-positive and typically appear as straight or slightly curved rods. In some cases, they may resemble branching structures akin to fungi.
Key Characteristics
- Acid fastness: This characteristic is due to the presence of mycolic acids in the cell wall and the integrity of the cell wall.
- Guanine plus cytosine (G + C) content: The G +C content of Mycobacteria DNA ranges from 61 to 71 mol%.
- Classification
- M. tuberculosis complex
- M. leprae
- Non-tuberculous mycobacteria (NTM)
Mycobacterium tuberculosis Complex
The M. tuberculosis complex comprises closely related species responsible for tuberculosis in humans:
- M. tuberculosis: The primary cause of tuberculosis in humans.
- Other species include: M. bovis, M. caprae, M. africanum, M. microti, M. pinnipedii, and M. canetti.
Pathogenesis
Tuberculosis (TB) is a serious disease affecting about one third of the world's population. Out of those infected, 5–10% will develop the actual disease. Notably, India has a significant burden, contributing to 25% of all global TB cases, with a prevalence rate of 256 and an incidence rate of 185 cases per 100,000 people.
Mode of Transmission
- Inhalation of tiny droplets (< 5–10 µm size) released by individuals with pulmonary tuberculosis.
- Inoculation (rare): Contact with infected skin.
- Ingestion (rare): Swallowing infected sputum (especially in infants) or drinking unpasteurized milk.
Risk Factors for Transmission and Disease Progression
- Sputum positive patients are more contagious.
- Bacillary load: A minimum of 104 bacilli/ml in sputum is necessary for effective transmission.
- Adult patients with cavitary lesions in the lungs have a higher bacillary load.
- Overcrowding in poorly ventilated spaces increases the risk.
- Low immunity: Individuals with weakened immune systems, like those with HIV, are at greater risk.
- Other health conditions: Issues such as post-silicosis, post-transplant complications, chronic kidney disease, diabetes, and substance abuse can increase risk.
- Age: Late adolescence and early adulthood are more vulnerable periods.
- Gender: Women aged 25–34 are at higher risk, while older men have a greater risk.
Pathogenic Events Sequence
- Droplet nuclei containing TB bacilli are inhaled from infected individuals.
- The majority are trapped in the upper airways and expelled by the action of cilia; usually, less than 10% of the smallest droplets reach the alveoli.
- Adhesion occurs when mycobacterial lipoarabinomannan (LAM) binds to receptors on macrophages.
- Phagocytosis is facilitated by complement (C3b) which helps in the uptake of bacteria by macrophages.
- Survival within macrophages is achieved through bacterial lipoarabinomannan, which prevents the fusion of phagosomes and lysosomes by disrupting intracellular signaling.
Clinical Manifestations
Tuberculosis (TB) can be classified into two main forms: pulmonary, which accounts for 80% of cases, and extrapulmonary, comprising 20% of cases.
Pulmonary Tuberculosis
Pulmonary tuberculosis is further divided into Primary Pulmonary Tuberculosis (PTB) and Post-primary (or Secondary) PTB.
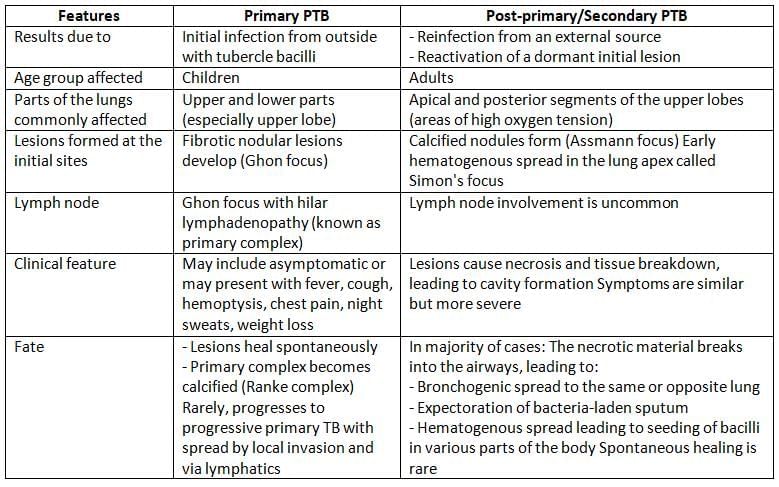
Extrapulmonary Tuberculosis (EPTB)
Extrapulmonary tuberculosis (EPTB) occurs when tubercle bacilli spread through the bloodstream to various organs. Although EPTB accounts for around 15% to 20% of all TB cases, this proportion is significantly higher in individuals with HIV, ranging from 20% to 50%.
The most commonly affected areas in EPTB, listed in order of frequency, are:
- Tuberculous lymphadenitis: This is the most common type of EPTB, representing 35% of cases. The posterior cervical and supraclavicular lymph nodes are the most frequently affected locations.
- Pleural tuberculosis: Accounting for 20% of EPTB cases, this condition typically manifests as a pleural effusion.
- Tuberculosis of the upper airways: This includes infections of the larynx, pharynx, and epiglottis.
- Genitourinary tuberculosis:
- Renal tuberculosis
- Genital tuberculosis: In women, the fallopian tubes and endometrium are often affected, leading to infertility, while in men, the epididymis is the most common site.
- Skeletal tuberculosis: This form of tuberculosis affects weight-bearing joints, with the spine being the most commonly affected area. Pott’s disease, also known as tuberculous spondylitis, is prevalent, along with infections of the hips and knees.
- Tuberculosis of the central nervous system (CNS): More common in children, this includes conditions such as tuberculous meningitis and tuberculoma.
- Gastrointestinal tuberculosis: The terminal ileum and cecum are the most frequently involved sites. Transmission can occur through swallowing sputum, blood spread, or consuming cow’s milk contaminated with Mycobacterium bovis.
- Tuberculous lymphadenitis is the most prevalent form of EPTB, constituting 35% of all cases. The posterior cervical and supraclavicular lymph nodes are the most commonly affected areas.
- Tuberculous pericarditis
- Tuberculous skin lesions:
- Scrofuloderma: This condition occurs when tuberculosis spreads to the skin from nearby lymph nodes, resulting in skin lesions.
- Lupus vulgaris: Characterized by the formation of apple jelly nodules on the face, particularly in women, this skin condition is caused by tuberculosis.
- Miliary or disseminated tuberculosis: This form of tuberculosis arises when tubercle bacilli spread through the blood, leading to the development of small granulomatous lesions resembling millet seeds in various organs. It is more commonly observed in individuals with HIV.
- HIV-associated tuberculosis: Tuberculosis is the most prevalent opportunistic infection in people with HIV, affecting 70% to 80% of individuals with the virus. In these cases, EPTB is more common than pulmonary TB.
Laboratory Diagnosis of Tuberculosis
Diagnosis of Active Tuberculosis
- Specimen collection:
- For pulmonary TB, specimens include sputum (2 samples: one spot and one early morning) and gastric aspirate in children.
- In EPTB, specimens vary based on the affected site.
- Digestion, decontamination, and concentration of specimen:
- Petroff’s method (4% NaOH)
- NALC (N-acetyl-L-cysteine) + 2% NaOH.
- Acid-fast staining by Ziehl Neelsen (ZN) technique:
- Procedure:
- Smear is covered with primary stain, strong carbol fuchsin for 5 minutes with intermittent heating.
- Decolorisation with 25% sulfuric acid for 3 minutes.
- Counterstaining with methylene blue for 1 minute.
- Advantages: Smear microscopy is quick, simple to perform in peripheral labs, and cost-effective.
- Disadvantages:
- Low sensitivity: Detection limit is 10,000 bacilli/ml of sputum.
- Viability of bacilli cannot be determined.
- Reporting: M. tuberculosis appears as long, slender, beaded, less uniformly stained, red-coloured acid-fast bacilli.
- RNTCP grading: Useful for assessing the infectiousness of the patient: the higher the grade, the more infectious the patient. Smear-negative patients are less infectious.
- Procedure:
- Other staining methods:
- Kinyoun’s cold acid-fast staining.
- Auramine phenol technique: a fluorescent staining method.
- Conventional culture media:
- Advantage: Culture is more sensitive with a detection limit of 10-100 viable bacilli.
- Disadvantage: It is time-consuming, needing a minimum of 8 weeks of incubation.
- Examples of media:
- Solid media:
- Egg-based: Lowenstein Jensen (LJ), Dorset egg media, Petragnani media. Lowenstein Jensen (LJ): M. tuberculosis forms rough, tough, and buff colonies, recommended by RNTCP.
- Agar-based: Middlebrook 7H11 and 7H10, preferred for isoniazid-resistant strains of M. tuberculosis.
- Liquid media: Middlebrook 7H9, Dubos, Proskauer, Sula, and Sauton media.
- Solid media:
- Automated culture methods: These systems continuously monitor growth and detect it faster (2-3 weeks); however, they are expensive:
- Examples include BACTEC, MGIT, and BACT/Alert.
- BACTEC MGIT: Uses an oxygen-sensitive fluorescent compound to detect:
- Mycobacterial growth
- Resistance to first-line anti-tubercular drugs.
- Biochemical identification: Positive for niacin test, nitrate reduction test, pyrazinamidase test, and resistant to TCH.
- Serology:
- Antigen detection—LAM and antigen-5 detection by ELISA.
- Antibody detection—not useful in endemic areas.
- Molecular methods:
- PCR detects the IS6110 gene and other genes like 65 KDa and 38 KDa.
- Line probe assay: Detects drug resistance from samples (takes one day), but only from smear-positive cases. It is less sensitive for smear-negative cases because it is based on hybridisation techniques.
- Gene Expert: Detects growth and resistance to rifampicin. It takes very little time (2 hours). It is a cartridge-based nucleic acid amplification technique (based on real-time PCR). Pleural biopsy is a better specimen than pleural fluid for Gene Expert.
- Animal pathogenicity: Using guinea pig and rabbit
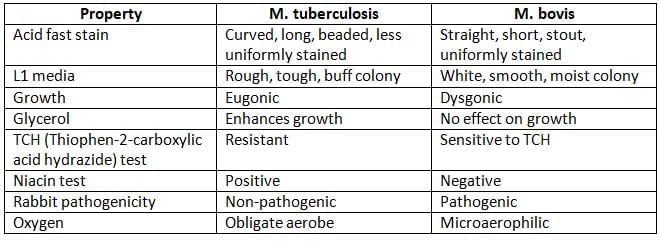
Diagnosis of EPTB:
EPTB specimens are paucibacillary; hence smear microscopy is less sensitive.
- Molecular methods are more effective.
- Pleural fluid: Shows elevated ADA (adenosine deaminase) and (IFN)-γ levels.
- Renal tuberculosis:
- Bacilli excretion in urine is intermittent; hence, 3–6 consecutive early morning urine samples are collected, centrifuged, and the sediment is processed.
- Acid alcohol is used as a decolouriser.
- CSF examination: Shows cobweb coagulum on standing, increased CSF pressure, protein, and chloride; while glucose levels are decreased.
Diagnosis of Latent Tuberculosis
It is diagnosed by demonstration of delayed or type IV hypersensitivity reaction against thetubercle bacilli antigens.
A. Tuberculin Test
Discovered by Von Pirquet in 1907.
- Antigens Used:
- Old tuberculin (OT): A basic mix of tubercle bacilli used previously.
- Purified protein derivative (PPD): A refined version of the active tuberculo protein, made by Seibert.
- Dosage: Measured in tuberculin units (TU). One TU equals 0.01 ml of OT or 0.00002 mg of PPD.
- Procedure:
- Mantoux test: The most common method. 0.1 ml of PPD with 1 TU is injected under the skin on the inner arm.
- Heaf and Tine multiple puncture tests: These methods are no longer used.
- Reading:
- Induration with surrounding erythema appears within 48 to 72 hours. If the width of the induration is:
- 10 mm or more: This is considered a positive result (known as tuberculin reactors).
- 6 to 9 mm: This result is seen as equivocal or doubtful.
- Less than 5 mm: This indicates a negative reaction.
- Interpreting the Results:
- Adults: A positive result shows either a current or past infection with tubercle bacilli but does not confirm active disease. It serves as an epidemiological marker:
- To find prevalence, count all tuberculin reactors in a community.
- To find incidence, count new positive tests in a community.
- Children: A positive result often indicates an active infection and is used as a diagnostic tool.
False Positive Results
- The test may show a false positive if:
- There has been a BCG vaccination (after 8–14 weeks).
- There is a non-tuberculous mycobacteria (NTM) infection.
False Negative Results
- The test can give false negative results in cases such as:
- Very early or advanced tuberculosis (TB).
- Miliary TB and post-measles.
- People with low immunity, including those infected with HIV.
Two-Step Testing: In adults, tuberculin sensitivity can decrease over time, becoming negative after several years. Repeating the test 1–2 weeks after the first test can create a booster effect, leading to a strong positive reaction (greater than 20 mm).
B. Interferon Gamma Release Assay (IGRA)
- Procedure: In the Interferon Gamma Release Assay (IGRA), sensitized T lymphocytes from individuals suspected of having tuberculosis are exposed to specific antigens derived from Mycobacterium tuberculosis , such as CFP10 (culture filtrate protein) and ESAT6 (early secreted antigenic target-6). This exposure leads to the release of high levels of interferon-gamma (IFNγ), indicating an immune response to the antigens.
- In Vitro Test: IGRA is an in vitro test, meaning it is conducted outside the body in a controlled laboratory environment. One of the commonly used formats for IGRA is the QuantiFERON-TB Gold assay, which employs enzyme-linked immunosorbent assay (ELISA) techniques to measure the levels of IFNγ released in response to the antigens.
- Advantage of IGRA: One of the significant advantages of IGRA is its high specificity. There are no false positive conditions associated with this test, making it a reliable tool for diagnosing latent tuberculosis infection. Unlike the Mantoux test, IGRA is not affected by prior BCG vaccination or infections with non-tuberculous mycobacteria, reducing the likelihood of false positive results.
Treatment
Anti-tubercular drugs are divided into two categories:
- First-line drugs: These are the primary medications used to treat tuberculosis and include:
- Isoniazid (H)
- Rifampin (R)
- Pyrazinamide (Z)
- Ethambutol (E)
- Streptomycin (S)
- Second-line drugs: These are used when first-line drugs are not effective or suitable and include:
- Ethionamide
- Cycloserine
- Macrolides such as Clarithromycin
- Quinolones like Ofloxacin and Ciprofloxacin
- Aminoglycosides such as Kanamycin, Capreomycin, and Amikacin
Treatment Strategies
- Multidrug therapy: This involves a combination of drugs and is usually prescribed for a short course lasting 6 months. For individuals who have been treated before, the duration may extend to 8 months.
- Two-phase chemotherapy: This strategy includes:
- Intensive phase: Lasting 2 to 3 months, where the treatment is more aggressive.
- Continuation phase: Lasting 4 to 5 months, focusing on completing the treatment.
- DOTS strategy: Recommended by the Revised National Tuberculosis Control Programme (RNTCP) and the World Health Organization (WHO), this strategy involves directly observed treatment to ensure adherence to the medication regimen.
- Treatment regimens: Category I and II regimens are followed according to the guidelines set by RNTCP, ensuring standardized and effective treatment protocols.
Drug Susceptibility Testing (DST)
Phenotypic Methods:
- Proportion Method: Considered the gold standard for testing drug susceptibility.
- Automated Systems: Such as BACTEC MGIT, widely used in contemporary practice.
- Absolute Concentration Method: method for determining drug susceptibility.
- Resistance Ratio Method: Another approach for testing drug resistance.
Molecular Methods:
- Involve techniques like PCR-based assays to detect drug-resistant genes in M. tuberculosis.
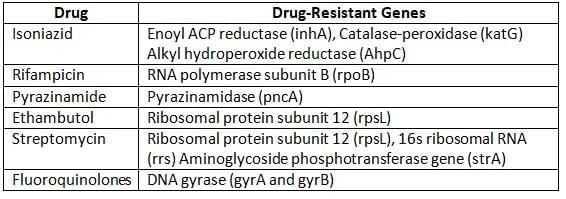
Understanding Multidrug Resistant Tuberculosis (MDR-TB)
MDR-TB refers to a type of tuberculosis that is resistant to two powerful first-line antibiotics: isoniazid and rifampicin. This resistance can occur with or without additional resistance to other first-line TB drugs.
Epidemiology of MDR-TB
- The World Health Organization (WHO) reports that a significant 60% of global MDR-TB cases are found in BRICS countries: Brazil, Russia, India, China, and South Africa.
- India holds the highest number of MDR-TB cases worldwide.
- In India, MDR-TB accounts for approximately 2.8% of all new TB cases and 12–17% of cases requiring re-treatment.
Treatment for MDR-TB
- Treatment for MDR-TB is carried out under the DOTS-Plus programme.
- This programme involves a complex regimen of second-line drugs, which is divided into two phases:
- Intensive Phase: This phase lasts for 6 to 9 months and includes 6 different drugs.
- Continuation Phase: Following the intensive phase, this phase lasts for 18 months and includes 4 different drugs.
Extensively Drug-Resistant Tuberculosis (XDR-TB)
- Definition: XDR-TB is a severe form of tuberculosis that arises from multidrug-resistant TB (MDR-TB) cases that also show resistance to:
- Fluoroquinolones, which are a class of antibiotics that include drugs like ofloxacin and levofloxacin.
- At least one injectable aminoglycoside antibiotic, such as kanamycin, amikacin, or capreomycin.
- Epidemiology: In the United States, about 3% of MDR-TB cases are identified as XDR-TB. The precise number of XDR-TB cases in India is not well-documented, but it is estimated that 2–6% of MDR-TB treatment failures could be due to XDR-TB.
- Treatment: Treating XDR-TB is extremely difficult because the disease progresses rapidly and is associated with high mortality rates.
Prophylaxis by BCG Vaccine (Bacillus Calmette Guerin)
The BCG vaccine was developed by Calmette and Guerin in 1921 by weakening the M. bovis strain through extensive subculturing. The strain was subcultured 230 times over 13 years in a glycerol bile potato medium.
- BCG Strain: The original strain used is a live weakened form of M. bovis.
- Although the same strain is still in use, different maintenance methods in various vaccine labs have led to the development of many substrains over the years.
- In India, the WHO recommends using the Danish 1331 strain of BCG, which is produced at the Central BCG Laboratory in Guindy, Chennai.
- Reconstitution: BCG comes in a freeze-dried form and must be mixed with normal saline before being given.
- Distilled water should never be used, as it can cause irritation.
- Once mixed, it should be administered within one hour.
- Administration of BCG:
- Dose and strength: 0.1 ml, which contains 0.1 mg TU.
- Do not use alcohol to clean the skin.
- Site: The injection is given above the left deltoid muscle.
- Route: The vaccine is injected intradermally using a 26-gauge tuberculin syringe.
- Phenomena after BCG: If the injection is done correctly,
- 6–12 weeks: A small permanent scar (4–8 mm in size) will appear at the injection site.
- 8–14 weeks: The Mantoux test will show a positive result.
- If too much is given, the scar may become larger and irregular.
- Protection:
- Efficacy: Studies have shown that BCG's effectiveness varies between 0–80%.
- Duration of immunity: It lasts for about 15–20 years.
- While BCG may not completely prevent tuberculosis infection, it provides significant protection for infants and young children against severe forms of the disease, such as meningitis and disseminated tuberculosis.
- Complications following BCG:
- The most common complications include ulceration at the vaccination site and swelling of nearby lymph nodes.
- Rare complications can include keloids, lupus lesions, and osteomyelitis.
- Very rarely, cases of non-fatal meningitis and progressive tuberculosis, as well as disseminated BCG infection (known as 'BCGitis'), have been reported in individuals with weakened immune systems.
- Indications for BCG:
- Direct BCG: BCG is administered to newborns shortly after birth, a common practice in many developing countries, including India. If not given at birth, it can be administered up to 2 years of age.
- Indirect BCG: BCG is given after conducting a tuberculin test.
- Contraindications to BCG:
- Children who are HIV positive.
- Children born to mothers who tested positive for AFB.
- Children with weakened immune systems.
- Children with generalized eczema.
- Pregnant women.
- Other uses of BCG:
- BCG may provide some protection against leprosy and leukemia.
- It has been studied as an additional treatment for cancers like bladder carcinoma (specifically the Onco TICE strain of BCG).
- Some studies suggest BCG might be more effective than PPD for tuberculin tests.
Non-Tuberculous Mycobacteria
Non-Tuberculous Mycobacteria (NTM), also referred to as atypical mycobacteria or Mycobacteria other than tubercle bacilli (MOTT), are microorganisms found in birds, animals, soil, and water. Although they are commonly present in these environments, NTM have the potential to cause infections in humans.
Runyon’s Classification of Non-Tuberculous Mycobacteria (NTM)
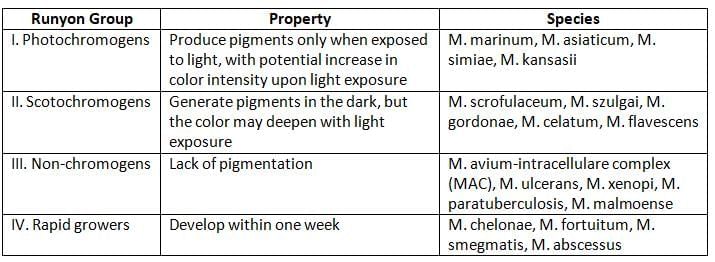
- NTM can be distinguished from MTB complex by:
- Being resistant to paranitrobenzoic acid (PNB), but sensitive to thiophen-2-carboxylic acid hydrazide (TCH).
- Testing positive for aryl sulfatase.
- Showing a strong positive reaction for catalase.
- Not causing disease in guinea pigs, but can cause illness in mice.
- Being resistant to antitubercular drugs.
Diseases from Nontuberculous Mycobacterium
Photochromogenic (Produce Pigments Only in Light)
- M. marinum: This bacterium is linked to various infections, such as:
- Swimming pool granuloma or fish tank granuloma.
- Tendonitis and tender nodules that may spread in a sporotrichoid pattern.
- M. asiaticum: This bacterium has a limited association with pulmonary disease, and further research is needed to understand its role better.
- M. simiae: This bacterium is characterized by a positive niacin test and the ability to produce pulmonary lesions.
- M. kansasii: This bacterium is known to cause chronic pulmonary disease that resembles tuberculosis.
Scotochromogens (Pigment Production in Light and Dark)
- M. scrofulaceum causes scrofula (cervical lymphadenitis) in children.
- M. gordonae is often found in tap water and is generally harmless.
- M. szulgai has the potential to cause lung disease and bursitis in some cases.
- M. celatum is an uncommon cause of lung infections.
Nonphotochromogens (No Pigment Production)
- M. avium-intracellulare complex (MAC) comprises two related bacteria, M. avium (Battey bacillus) and M. intracellulare
- The potential effects of MAC include:
- Lymphadenitis (swollen lymph nodes)
- Respiratory infections
- Widespread infections
- M. xenopi has been detected in hospital water and associated with outbreaks.
- M. ulcerans is a waterborne skin pathogen primarily found in tropical regions of Africa, America, and Southeast Asia, leading to conditions such as:
- Buruli ulcer
- Osteomyelitis (bone infection)
- Limb deformities
- M. malmoense has the potential to cause lung disease and, less frequently, lymphadenitis.
- M. paratuberculosis, also known as Johne’s bacillus, is associated with Crohn’s disease, although further research is necessary to establish this link.
Rapid Growers (Grow within 1 Week of Incubation)
- M. fortuitum and M. chelonae are known to cause post-trauma injection abscesses and infections related to catheters.
- M. abscessus has the potential to cause pulmonary infections.
- To identify rapid growers, the Arylsulfatase test is used, which yields a positive result for these organisms.
Mycobacterium leprae
Mycobacterium leprae, also referred to as Hansen’s bacillus, is the causative agent of leprosy. This ancient condition has often been linked with social stigma due to myths and the visible deformities it can induce.
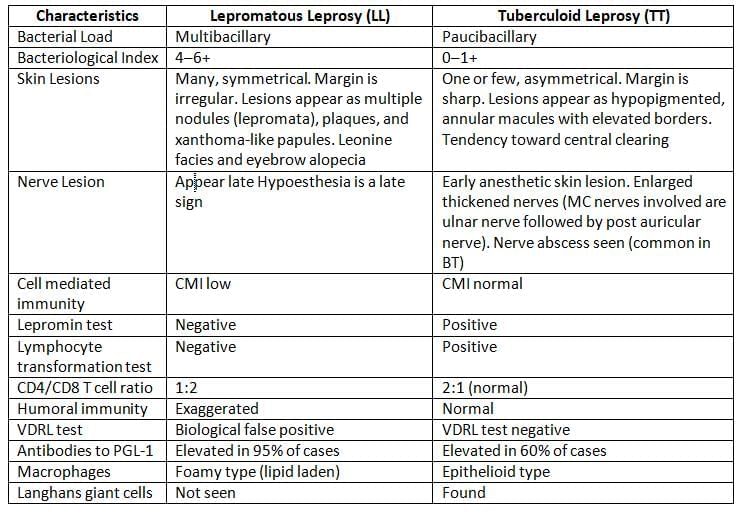
Clinical Manifestations and Classification
- Slick Lesions of Tuberculoid Leprosy:
- One or few, asymmetrical lesions
- Sharp margins
- Appear as hypopigmented, annular macules with elevated borders
- Tendency for central clearing
- Incubation period:Leprosy has a long incubation time, usually averaging 3–5 years.
- This is due to the lengthy generation time of lepra bacilli (12–13 days).
- Lepromatous leprosy (LL) cases typically have a longer incubation period than tuberculoid leprosy (TT); LL averages 5–7 years, while TT averages 3–5 years.
Clinical Classification of Leprosy:
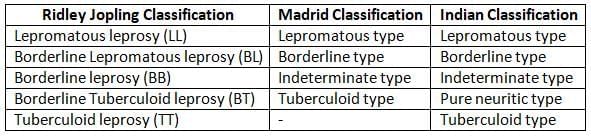
Leprosy is a bipolar disease. Under any classification scheme, lepromatous and tuberculoid cases are the two extreme poles of the disease (described in table). Other varieties are:
- Borderline type:
- Patients show features that are a mix between the tuberculoid and lepromatous types.
- They can switch between these two types based on treatment or changes in their body's defenses.
- Indeterminate type:
- This type includes early cases that are not stable.
- Patients may have one or two light-colored patches on the skin and clear signs of sensory loss.
- These cases do not show bacteria in tests.
- Pure neuritic type:
- This type involves nerve damage without any skin lesions.
- These cases also do not show bacteria in tests.
Deformities in Leprosy:
About 25% of untreated leprosy cases develop deformities over time. These deformities can result from:
- Nerve injury causing muscle weakness or paralysis
- The disease process itself, leading to facial deformities or loss of eyebrows
- Secondary infections or injuries, such as ulcers
Common Deformities Include:
- Face: Leonine facies (thickened skin and facial deformities), sagging face, loss of eyebrows and eyelashes, corneal ulcers
- Hands: Claw hand (due to nerve damage), wrist drop (due to radial nerve injury)
- Feet: Foot drop (due to peroneal nerve injury), clawing of toes, foot inversion, plantar ulcers
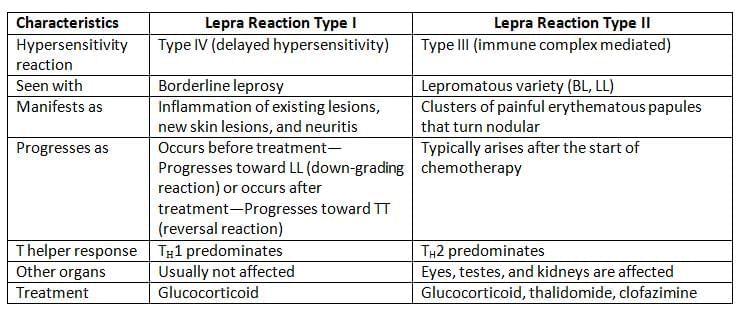
Lepra Reactions
Leprosy, despite being a chronic condition, can experience sudden allergic flare-ups during its course, referred to as lepra reactions. These reactions are classified into two types: Type I and Type II.
Epidemiology
Source of Infection: Multibacillary cases (LL and BL) are the primary source of infection.- Mode of Transmission:
- Nasal droplet infection (most common mode).
- Contact transmission.
- Breast milk, vertical transmission, and tattooing.
- Communicability: Leprosy has low communicability, requiring prolonged close contact for transmission.
Geographical Distribution
Leprosy is predominantly found in developing countries across Asia, Africa, Latin America, and the Pacific.
India reports the highest number of cases globally, although the incidence rate is declining:
- As of 2012, the prevalence rate in India is approximately 0.68 cases per 10,000 population.
- 32 states and Union Territories (UT) have achieved leprosy elimination status, with fewer than 1 case per 10,000 population.
- Chhattisgarh and Dadra and Nagar Haveli are the only regions exceeding the elimination threshold, while Bihar is nearing the borderline.
Laboratory Diagnosis
Smear Microscopy
Smear microscopy is a diagnostic technique used to detect acid-fast bacilli (AFB) in skin lesions, which is indicative of certain infections like leprosy. The procedure involves collecting samples from various sites and performing specific staining techniques to visualize the bacilli.
- Sample Collection: total of six samples are collected from the following sites:
- Skin Samples: Collected from the edge of the lesion on the forehead, cheek, chin, and buttock using the slit skin smear technique.
- Ear Lobe Sample: Collected using the slit skin smear technique.
- Nasal Mucosa Sample: Collected by nasal blow and scraping.
- Biopsy: In some cases, a biopsy may be needed from thickened nerves and nodular lesions.
- Staining Technique: Acid-fast staining is performed using the Ziehl-Neelsen technique, which involves the use of carbol fuchsin and an acid-alcohol decolouriser.
- Microscopic Appearance: Under the oil immersion objective, red acid-fast bacilli can be observed, either singly or in groups, bound together by a lipid-like substance called glia, forming structures known as globi.
- Live Bacilli: Uniformly stained with parallel sides and round ends, approximately five times longer than their width.
- Dead Bacilli: Less uniformly stained with a fragmented and granular appearance.
- Grading of Smear:
- Bacteriological Index (BI): Based on the total number of bacilli (live and dead) seen per oil immersion field.
- Morphological Index (MI): Percentage of uniformly stained bacilli out of the total number counted, serving as a better marker for monitoring treatment response.
- SFG Percentage: Percentage of solid, fragmented, and granular rods.
Mouse Foot Pad Cultivation
M.leprae is not cultivable either in artificial culture media or in tissue culture. The only certain way to cultivate M.leprae is by inoculating the specimens into:
- Nine banded armadillo and
- Foot pad of mice.
Antibody Detection
- FLA-ABS (Fluorescent Leprosy Antibody Absorption test)
- ELISA detecting IgM antibodies to PGL-1 (phenolic glycolipid-1) antigen of M.leprae
Test for Detecting CMI (Lepromin Test)
- The Lepromin test shows a delayed immune response and a functioning cell-mediated immunity (CMI) against the leprosy antigen.
- Procedure:
- A dose of 0.1 ml of lepromin antigen is injected into the skin of the forearm.
- The results are checked twice: once after 48 hours and again after 21 days.
- Early reading (Fernandez reaction) at 48 hours:
- If there is swelling and redness measuring more than 10 mm, it suggests a delayed immune response, indicating previous exposure to leprosy bacteria.
- However, this does not confirm an active infection.
- Late reading (Mitsuda reaction) at 21 days:
- A bump larger than 5 mm appears at the injection site, which may later develop into an ulcer.
- This result indicates that the patient's CMI is functioning well.
- Uses of the Lepromin test:
- The late reaction helps measure CMI, which can be useful for:
- Classifying leprosy lesions: In tuberculoid (TT) patients with good CMI, the test is usually positive. In lepromatous (LL) patients, the test is negative, indicating low CMI.
- Assessing prognosis: A strong CMI (as seen in TT patients) suggests a better outlook for recovery.
- Evaluating resistance to leprosy: Individuals who are negative for lepromin are at a greater risk of developing multibacillary leprosy compared to those who test positive.
- The late reaction helps measure CMI, which can be useful for:
Treatment
Because of risk of developing resistance, WHO recommends multidrug therapy (MDT) for treatment of leprosy:
- Recommended Drugs: Dapsone, rifampicin, and clofazimine.
- Alternate Drugs: Ethionamide, quinolones (ofloxacin), minocycline, and clarithromycin.
- WHO treatment regimens are administered based on the clinical type of leprosy.
Clinical classification of leprosy and WHO treatment regimens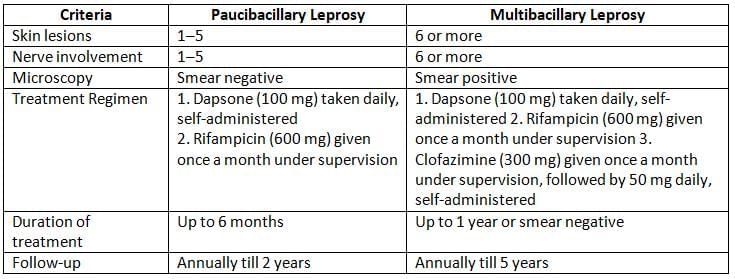
Prevention of Leprosy
- Finding active cases and providing effective treatment are the most important measures.
- BCG Vaccine: Trials with BCG have shown effectiveness of 28–60%.
- Chemoprophylaxis: Dapsone can be given to contacts of TT patients, but not to LL patients.
- Hospitalised patients generally do not need isolation, as transmission usually requires prolonged contact.
Leprosy Elimination Efforts
The National Leprosy Eradication Programme in India focuses on two main goals:
- The elimination of leprosy, which has already been accomplished in most parts of India, except for Chhattisgarh and Dadra and Nagar Haveli.
- The decentralization of services by integrating them into the general healthcare system.
Although India has reached the elimination target of less than 1 case per 10,000 people, it still has the highest number of leprosy cases globally. Therefore, the long-term goal is to fully eradicate leprosy from the country.
Challenges in Eradicating Leprosy:
- Long and variable incubation period
- Uncertain transmission methods
- Higher number of subclinical cases
- Low immunity in patients with LL
- Lack of an effective vaccine
- Bacterial resistance
- Complex disease spectrum
- Poor patient compliance due to long treatment durations
- Social issues
National Institutes of Leprosy:
- National JALMA Institute of Leprosy and Other Mycobacterial Diseases, Agra
- CLTRI (Central Leprosy Training and Research Institute), Chengalpattu, Tamil Nadu
|
75 docs|5 tests
|
FAQs on Mycobacteria Chapter Notes - Microbiology - NEET PG
| 1. What are the key characteristics of Mycobacteria and how do they contribute to pathogenesis? |  |
| 2. What are the common clinical manifestations of tuberculosis? |  |
| 3. How is tuberculosis diagnosed in the laboratory? |  |
| 4. What is the treatment regimen for tuberculosis and how are drug-resistant strains addressed? |  |
| 5. How does the BCG vaccine work in the prevention of tuberculosis? |  |














