Myxoviruses and Rubella Chapter Notes | Microbiology - NEET PG PDF Download
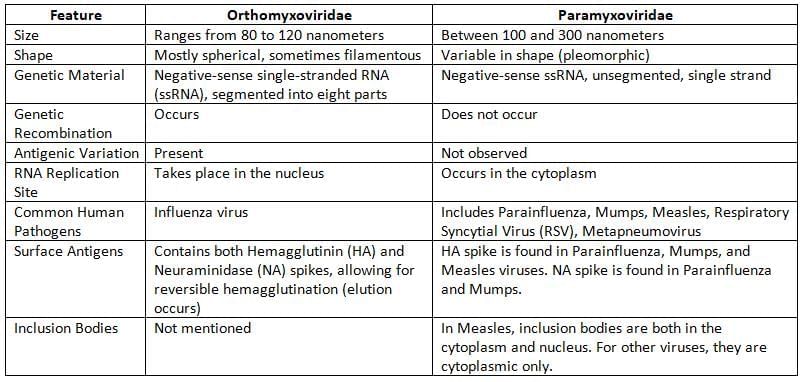
Orthomyxoviruses
Influenza Virus
General Properties
- Influenza virus has a spherical shape with helical symmetry.
- Its viral RNA consists of eight segments of negative sense single stranded RNA.
- RNA replication happens in the nucleus, unlike most RNA viruses which use the cytoplasm.
- Viral proteins include eight structural ones (PB1, PB2, PA, NP, HA, NA, M1, M2) and two nonstructural (NS1, NS2).
Antigens and Typing
Influenza has two glycoprotein antigens in its lipid envelope: HA (Hemagglutinin) and NA (Neuraminidase).
- Hemagglutinin (HA): Attaches to mucin or sialic acid receptors on red blood cells, causing them to clump (hemagglutination). It also attaches to host cells to help virus entry. Antibodies against HA provide protection.
- Neuraminidase (NA): Fewer in number, acts as a sialidase enzyme that breaks down sialic acid receptors on red blood cells, aiding in: displacing HA from red blood cells to reverse hemagglutination (elution), helping release virus from infected cells, and allowing virus to move through mucin in respiratory tract.
- For example, HA's role in entry can be seen in how blocking it with antibodies prevents infection, illustrating its importance in protection.
Typing
- Three genera based on Nucleoprotein (NP) and Matrix (M) proteins: Influenza A (most common for outbreaks, epidemics, and the only one for pandemics), Influenza B (rarely seen), Influenza C (not circulating now).
- Subtypes: For Influenza A, based on HA and NA, there are 16 H subtypes (H1 to H16) and 9 N subtypes (N1–N9). Influenza B and C have subtypes but not designated.
- Influenza Typing: Influenza A is most common for outbreaks/epidemics and only for pandemics, Influenza B is rarely seen, Influenza C is not circulating now.
Antigenic Variation
Influenza A and to a lesser degree B show antigenic variation in two forms: shift and drift. Type C is stable.
- Antigenic Shift: In Type A, leads to pandemics and major epidemics, due to genetic reassortment, occurs every 10–20 years.
- Antigenic Drift: In Type A and less in B, leads to periodic epidemics and sporadic cases, due to point mutations, occurs every 2–3 years.
- For example, antigenic shift is like a major overhaul where virus segments mix from different sources, such as in pigs, leading to new strains humans have no immunity against, causing pandemics.
Currently circulating strains
- Type A/H1N1 (2009 Pandemic flu strain).
- Type A/H3N2 (Seasonal flu strain).
- Type A/H5N1 (Avian flu strains).
- Type B.
Clinical Features
- Incubation period: 18–72 hours.
- Reservoir: Animals, birds, and humans.
- Most common manifestation: Mild flu-like illness (upper respiratory tract infection).
- Most common complication: Bacterial pneumonia (S. aureus more than pneumococci and H. influenzae) is more frequent than viral pneumonia; Reye’s syndrome common with Type B (fatty liver after aspirin).
Laboratory Diagnosis
- Specimen collection: Nasopharyngeal swab using polyester or Dacron swabs.
- Transported in viral transport media, kept at 4°C up to 4 days, then at -70°C.
- Isolation of virus: In embryonated eggs and primary monkey kidney cell lines.
- Egg inoculation: Amniotic cavity supports Influenza A, B, C; Allantoic cavity supports only Influenza A; Growth detected by hemagglutination with fowl and guinea pig RBC; Type A agglutinates guinea pig RBC, Type C fowl RBC at 4°C, Type B both.
- Antigen detection from nasopharyngeal cells by direct IF test.
- Antibody detection: Fourfold rise in titer is significant; Hemagglutination inhibition test (HAI); Neutralization test is most specific and best predictor of susceptibility but time-consuming; ELISA more sensitive than others.
- Molecular methods: Reverse transcriptase PCR (RT-PCR) is most sensitive, type specific; Real time RT-PCR quantitates viral load.
Vaccine
Killed Vaccine:
- Produced in the allantoic cavity of eggs, it includes HA antigen (15 μg per dose).
- Administered in two doses via intramuscular (IM) route, with an effectiveness of 70-90%, lasting for 6-12 months.
- A single dose can be given by either IM or subcutaneous (SC) route, providing protection of about 50-80%, with immunity lasting for 6-12 months.
- Indication: Recommended annual inactivated influenza vaccination for high-risk groups, including:
- Children
- Older adults
- Individuals with underlying conditions like chronic lung, heart, kidney, liver, and central nervous system (CNS) issues
- People with low immunity (e.g., HIV)
- Pregnant women
- Contraindication: Not suitable for those allergic to eggs or with a history of severe reactions to previous doses.
- Types of Inactivated Vaccines: There are three types, all of which are effective:
- Whole Virus (WV) vaccine: Contains whole, inactivated viruses.
- Subvirion (SV) vaccine: Contains purified viruses that have been broken down using detergents.
- Surface Antigen Vaccines: Contain purified HA and NA glycoproteins.
Live Nasal Spray (Trivalent):
- It is a trivalent vaccine, containing:
- Influenza A (H1N1)
- Influenza A (H3N2)
- Influenza B
- Stimulates both local and systemic immunity, but not recommended for those with low immunity.
- Utilizes a cold-adapted strain.
- Indication: Recommended for all healthy individuals aged 2-49 years, but not advised for high-risk groups.
Treatment
Matrix M2 inhibitor (amantadine) and neuraminidase inhibitor (oseltamivir).
Sialic Acid Receptors Determines Pathogenicity
- Virus entry depends on sialic acid receptors on host cells, specific to HA.
- α 2–6 sialic acid receptors for human strains, abundant on upper respiratory tract but not lower, so mild upper infections, not pneumonia.
- α 2–3 sialic acid receptors for avian strains, abundant on bird intestine; In humans, few on lower tract, so hard to infect but can cause pneumonia if infected.
- Why pigs are common mixing vessels: Both α 2–3 and α 2–6 on pig respiratory epithelium, swine flu specific for both; Pigs infected by human, swine, avian strains, allowing reassortment.
- Sialic acid receptors determines pathogenicity of Influenza: α 2–6 specific for human influenza; α 2–3 specific for avian influenza; Both α 2–3 and α 2–6 found in pigs, hence pigs are most common mixing vessels.
- For example, in humans, the dominance of α 2–6 receptors explains why seasonal flu mostly stays in the upper airways, while avian strains, binding α 2–3, can go deeper if they infect, leading to severe pneumonia.
Avian Flu Infection in Humans
- Birds are the main carriers of influenza viruses.
- It is thought that all human pandemic strains have come from a mix of avian and human influenza viruses, and this mixing has happened in pigs.
- A/H5N1is the most common avian flu strain that has been present worldwide.
- Origin: It was first identified in Hong Kong in 1997 and has spread to many countries, including India.
- Transmission to humans: This virus spreads only from birds and requires close respiratory contact.
- Less morbidity: Since there is no transmission from human to human, the number of sick cases is low.
- More mortality: Avian flu strains are very deadly (due to the PB1F2 protein) and have a mortality rate greater than 60%.
- Clinical features: H5N1 avian flu strains are linked to higher rates of pneumonia (over 50%) and other serious symptoms like diarrhea and CNS involvement.
- Other avian flu strains that can infect humans include:
- A/H7N7 (from the Netherlands)
- A/H9N2 (from Hong Kong)
- A/H7N9 (which caused an outbreak in China in 2013)
- Laboratory diagnosis: This is done using real-time RT-PCR to detect specific HA and NA genes.
- Treatment: The recommended medication is oseltamivir (known as Tamiflu).
A/H1N1 2009 Flu
Caused recent pandemic, emerged California March 2009, spread worldwide including India; WHO declared pandemic June 11, 2009.
Epidemiology:
- Origin: Genetic reassortment of four strains (1 human + 2 swine + 1 avian), mixing in pigs.
- Not correctly called ‘swine flu’ as it's a reassortant of four strains.
- Transmission: Human to human, caused rapid spread.
- Less virulent (lacks PB1F2), so more morbidity but less mortality than H5N1.
- World situation: Post-pandemic except India and New Zealand with local transmission.
- India situation: Since 2009, 53,943 cases, 3,315 deaths; 2013: 708 cases, 132 deaths; 2015 outbreak: 33,761 affected, 2035 deaths (up to March 30, 2015), worst in Rajasthan, Gujarat, Maharashtra, Madhya Pradesh.
- H1N1 situation in India: Since 2009 about 53,943 cases and 3,315 deaths due to H1N1 were reported from India, out of which in 2013 alone nearly 708 cases with 132 deaths have occurred; However, a threatening outbreak of H1N1 started again in 2015 affecting 33,761 people with 2035 deaths (up to March 30th 2015). The worst hit states are Rajasthan, Gujarat, Maharashtra and Madhya Pradesh.
Clinical Feature:
- Uncomplicated: Most with mild URTI and diarrhea.
- Complicated/severe: Rare in high risk, with secondary bacterial pneumonia, dehydration, CNS, multiorgan failure.
Laboratory Diagnosis
- Real time RT-PCR detects and quantifies HA and NA genes.
Treatment:
- Resistant to amantadine; Neuraminidase inhibitors: Oseltamivir (Tamiflu) 75 mg twice daily for 5 days; Zanamivir (10 mg inhalational).
Prophylaxis:
- Vaccine: Killed injectable and live nasal spray for A/H1N1 2009.
- Chemoprophylaxis: Oseltamivir 75 mg once daily, duration based on setting.
Paramyxoviruses
Family including viruses like parainfluenza, mumps, measles, RSV causing respiratory and other issues.
Parainfluenza Viruses
- Human parainfluenza viruses major cause of lower respiratory disease.
- Common cold syndrome like rhinitis and pharyngitis is most common.
- Croup (laryngotracheobronchitis): With types 1 and 2, in older children.
- Pneumonia or bronchiolitis: Rare under 6 months, especially type 3.
- Otitis media most common complication.
- Reinfections common, no cross protection between serotypes.
- Human parainfluenza viruses: Most common agent of Croup (laryngotracheobronchitis).
Mumps Virus
- Mumps virus most common for parotid gland enlargement in children.
- Transmission via respiratory droplets, saliva, fomites.
- Incubation period about 19 days (7–23 days).
Clinical Manifestation
- Inapparent infection: Most common.
- Bilateral parotitis: Most common (70–90%), rarely other salivary glands.
- Epididymo-Orchitis next most common, unilateral mostly, infertility rare.
- Aseptic meningitis <10%, male predominance, self-limiting but deafness permanent.
- Oophoritis in about 5% women.
- Pancreatitis in 4%, may lead to diabetes.
- Atypical mumps: Parotitis absent in 10%, direct aseptic meningitis.
Epidemiology
- Endemic worldwide, sporadic year-round, peak winter-spring.
- Epidemics every 3–5 years, in unvaccinated overcrowded areas.
- Period of communicability: 1 week before to 1 week after symptoms.
- Source: Cases, no carrier state.
- Reservoir: Humans only.
- Age: Children 5–9 years most affected, more severe in adults.
- Immunity: One attack (vaccine or infection) lifelong.
- Secondary attack rate high (86%).
Prevention (Live Attenuated Vaccine)
- Jeryl Lynn strain recommended worldwide, others RIT 4385, Urabe, L-Zagreb.
- Prepared in chick embryo cell line.
- Available as: Trivalent MMR (measles-mumps-rubella), Quadrivalent MMR-V (plus varicella), Monovalent (not common).
- Schedule: Two doses MMR IM at 1 year and 4–6 years (before school).
- Efficacy about 90% after second dose, neutralizing antibodies in 95%.
Measles (Rubeola) Virus
- Acute, highly contagious childhood disease with fever, respiratory symptoms, rash.
- Transmission mainly respiratory route.
Clinical Manifestations
Incubation period about 10 days, shorter in infants, up to 3 weeks in adults.
- Fever first, on day-1 (10th day of infection).
- Koplik’s spots pathognomonic, after two days fever: White to blush spot (1 mm) with erythema, first on buccal mucosa near second lower molars, spread entire.
- Rash: Maculopapular dusky red after four days fever (14th day): First behind ears, spread face, arm, trunk, fade same order; Absent in HIV.
- Sequence: Incubation (10 days) → Fever (10th day) → Koplik’s spot (12th day) → rash (14th day).
Complications
- Secondary bacterial infections: Following measles, there is profound CMI suppression which in turn predisposes to various secondary bacterial infections.
- Otitis media and bronchopneumonia are the most common.
- Worsening of underlying tuberculosis with a false negative Mantoux test
- Complications due to measles virus itself
- Giant-cell pneumonitis in immunocompromised, and HIV infected people
- Acute laryngotracheobronchitis (croup)
- Diarrhea, leads to malnutrition including vitamin A deficiency
- CNS complications are rare but most severe
- Postmeasles encephalomyelitis
- Measles inclusion body encephalitis
- Subacute sclerosing panencephalitis (SSPE) is a slowly progressive disease characterized by seizures and progressive deterioration of cognitive and motor functions.
- SSPE belongs to group C slow virus infection, caused by a defective measles virus.
- Occurrence in 1 in 300,000 measles cases.
- SSPE typically occurs in persons infected with measles virus < 2 years of age.
- SSPE usually develops after 7-10 years of initial infection.
- It is fatal within 1-3 years of onset with mortality rate of 10-20%.
- High titer antibody in 1-3 year CSF is diagnostic.
Laboratory Diagnosis
- Cell lines: Monkey/human kidney or lymphoblastoid (B95-a), Vero/hSLAM CDC recommended.
- Cytopathic effect: Multinucleated giant cells (Warthin-Finkeldey) with intranuclear and intracytoplasmic inclusions.
- Cytopathic effect of Measles: Multinucleated giant cells (Warthin-Finkeldey cells) containing both intranuclear and intracytoplasmic inclusion bodies.
Live Attenuated Measles Vaccine
- Strains: All are derived from the original Edmonston strainthat was isolated in 1954, which includes:
- Schwartz strain (currently serves as the standard in many parts of the world)
- Edmonston-Zagreb strain
- Moraten strain
- Vaccine preparation: The vaccine is created using a chick embryo cell line.
- Reconstitution: The vaccine is provided in a lyophilized form and needs to be mixed with distilled water. It should be used within 4 hours after reconstitution.
- Storage: The vaccine is thermolabile, which means it must be kept at -20°C.
- Dosing: A single dose of 0.5 ml is given subcutaneously, containing more than 1000 infective viral units.
- Indication: It is administered at 9 months of age, as maternal antibodies fade away by this time, along with vitamin A supplements.
- However, it can also be given at 6 months during a measles outbreak. In this case, a second dose should be provided at 9 months.
- Side effects:
- A mild measles-like illness can develop in 15-20% of recipients. The vaccine virus does not spread in the community.
- Toxic shock syndrome can occur when a vaccine vial is contaminated with toxins from S. aureus.
Measures taken following exposure:
- Measles vaccine within 3 days; Vaccine IP 7 days vs natural 10 days.
- Immunoglobulin within 3 days, WHO dose 0.25 mg/kg.
- Not together, gap 8–12 weeks.
- Measures taken following exposure to measles patient: Measles vaccine or Ig can be given within 3 days; However, both should not be given together; At least 8–12 weeks of gap must be maintained.
Epidemiology
Endemic, epidemics every 2–3 years, late winter-early spring.
- Source: Cases only, no carrier.
- Reservoir: Humans only, no animal.
- Communicability: 4 days before to 4 days after rash.
- Secondary attack >90%.
- Age: Childhood; 6 months-3 years developing countries; >5 years developed/vaccinated.
- Immunity: No age immune without prior; Single serotype, lifelong after one attack; Infants protected to 6 months by maternal antibodies.
- Genotypes: 8 clades, 23 genotypes (WHO); Global B3 most common, India D8.
- Epidemic if susceptible children >40%.
- Recent outbreaks: 2014 Philippines, Vietnam.
Measles Eradication
- Possible with immunization.
- WHO strategy: ‘Catch up, Keep up and Follow up’ immunization.
- A catch-up campaign is a one-time initiative aimed at vaccinating all children aged between 9 months and 10 years, regardless of their previous vaccination history. The purpose of this campaign is to quickly lower the number of unprotected children in the community.
- Follow-up campaigns occur every 2 to 4 years after the catch-up campaigns. These are meant to vaccinate all children older than 9 months who were born after the last catch-up campaign.
Respiratory Syncytial Virus
- Clinical Manifestations
- Infants: RSV is the leading cause of lower respiratory infections in children under 1 year old, leading to conditions like bronchiolitis, pneumonia, and tracheobronchitis.
- Adults: RSV results in upper respiratory symptoms similar to those of the flu.
- Exacerbation: RSV can worsen asthma or COPD symptoms.
- Recurrent Infections: Though common, subsequent infections are usually milder, resembling a common cold.
- Laboratory Diagnosis
- Virus Isolation: HeLa and HEp-2 cell lines are the most effective for isolating the virus.
- Cytopathic Effect: A distinct effect, known as syncytium formation (which creates multinucleated giant cells), can be seen after about 10 days, leading to the name syncytial virus.
- Epidemiology
- Seasonality: RSV infections are more common during rainy weather in winter and spring.
- Age: The highest risk of infection is in infants aged between 6 weeks and 6 months.
- Subgroups: RSV has two main subgroups; infections from Subgroup A tend to result in more severe illness.
- Treatment
- Ribavirin: This is the preferred medication for severe RSV infections in infants. Its effectiveness in older children and adults is uncertain. It is usually given as an aerosol for 3 to 6 days.
Rubella
- Rubella is not a type of myxovirus, but it is included in this discussion because it has similar clinical features to measles.
- Rubella is commonly referred to as German measles.
- It is part of the Togaviridae family.
Epidemiology
- Source: Only cases, no carriers.
- Once infected: Lifelong immunity.
- In India, 40% reproductive age females susceptible.
- Communicability: -1 week to +1 week of rash.
- IP 2–3 weeks (14 days).
- Transmission: Droplet, contact, sexual, in-utero.
Clinical Manifestations in Adult
- Subclinical: 50%.
- Rash day 1 (face): Lasts 3 days.
- Lymphadenopathy (occipital, postauricular).
- Forschheimer spots: Pin-head petechiae on soft palate, uvula, with rash onset.
Congenital Rubella Syndrome
- Risk max in 1st trimester, negligible after 5th month.
- Permanent congenital defects
- Classical Triad:
- Ear defect: The most common issue is nerve deafness, often seen in congenital rubella syndrome.
- Ocular defects: The most frequent issue is salt-and-pepper retinopathy, followed by cataracts.
- Cardiac defect: The most common heart problem is patent ductus arteriosus (PDA), followed by pulmonary artery stenosis and ventricular septal defect.
- CNS defects:
- Conditions such as microcephaly, mental retardation, motor delays, and autism may occur.
- Classical Triad:
- Transient congenital changes:
- Possible changes include hepatosplenomegaly, bone lesions, intrauterine growth retardation (IUGR), and thrombocytopenia with petechiae (known as Blueberry muffin syndrome).
- Diagnosis:
- IgM testing at birth or persistent IgG levels that do not decrease by twofold per month.
- Virus isolation within six months.
- RT-PCR for detecting viral RNA.
Laboratory Diagnosis
- Most commonly used tests: Hemagglutination inhibition test (HAI) and ELISA
- Culture:
- Best specimen: Nasopharyngeal or throat swabs
- Preferred cell line: Cell lines from monkeys or rabbits can be utilized.
- Identification: Recognized through interference with Echovirus.
- Understanding serology in congenital rubella infection:
- IgM antibodies: These do not cross the placenta; finding them in a newborn indicates a diagnosis of congenital rubella infection.
- IgG antibodies: These cannot distinguish between antibodies from the mother and those from a true congenital infection. However, if IgG remains in the baby’s blood longer than expected after maternal IgG should fade, it can help confirm the diagnosis.
Rubella Vaccination (RA 27/3 Live Attenuated)
- Prepared from human diploid cell line, a single dose (0.5 ml) of the vaccine is given under the skin.
- The vaccine should not be given during pregnancy.
- Because it can cause birth defects, it is advised to avoid getting pregnant for at least 4 weeks (28 days) after getting vaccinated.
- Infants under 1 year should not receive the vaccine because their mother's antibodies may affect the vaccine's effectiveness.
- In India, the priority groups for the rubella vaccine include all women of reproductive age (the first priority group), followed by all children aged 1 to 14 years.
Daywise Appearance of Rashes
- 1st day–Rubella.
- 2nd day–Chickenpox.
- 3rd day–Smallpox.
- 4th day–Measles.
- 5th day–Parvovirus B19–Exanthem infectiosum.
- 6th day–HHV6–Exanthem subitum or Roseola infantum.
Vaccine Storage
- Deep Freezer: Polio, Measles (–20°C).
- Cold part (4°C), no freeze: DPT, Typhoid, TT, DT, BCG diluents.
- Vitamin A: Outside, room temperature.
- Most vaccines store 5 weeks in refrigerator 4–8°C.
- Open multidose discard: Within 1 hr (no preservative, most live); Within 3 hr or session end (with preservative).
|
75 docs|5 tests
|
FAQs on Myxoviruses and Rubella Chapter Notes - Microbiology - NEET PG
| 1. What are Myxoviruses, and how do they relate to the influenza virus? |  |
| 2. What are Paramyxoviruses, and what diseases do they cause? |  |
| 3. How does the live attenuated measles vaccine work? |  |
| 4. What is the impact of the respiratory syncytial virus (RSV) on infants and young children? |  |
| 5. Why is rubella vaccination important in public health? |  |















