Physical and Chemical Changes Chapter Notes | Chemistry Class 8 ICSE PDF Download
Introduction
Hello kids! Today we will learn about changes that happen around us. Some things change their shape, size, or color, but they stay the same inside. These are called physical changes. Some things change completely and become something new, like when we cook food. These are called chemical changes. We will see many examples, do fun activities, and learn how to tell if a change is physical or chemical. Let's start exploring!
Changes—A Review
Every day, we see many changes around us. Different things change in different ways. Some things change from one form to another.
- For example, an ice cube turns into water when it melts:
- When you keep an ice cube in the open, it changes into water.
- When you add salt to water, it becomes salty water.
- More examples of changes around us:
- Day changing into night: Sun goes down, and the moon comes up.
- Change of seasons: Weather changes from summer to winter.
- Digestion of food: Food breaks down in your tummy.
- A baby growing into an adult: A small baby grows into a big person.
- Curdling of milk: Milk turns into curd.
- Rusting of iron: Iron gets brown and rough.
- Burning of fuels: Fire burns wood or coal.
- Cooking of food: Food gets heated and becomes tasty.
- Fermentation: Tiny living things change food, like milk to curd.
- Ripening of fruits: Fruits become sweet and soft.
- Seeding growing into a plant: A small seed grows into a big plant.
Classification of Changes
- Changes are different types we see in the world.
- Some changes make things look different in shape, color, size, or position.
- Some changes make a new thing while the old thing goes away.
- Some changes are easy to change back, but some are hard to change back.
- Some changes happen fast, and some take a lot of time.
- We can put changes into different groups to understand them better.
- The groups are:
- Changes we can change back and changes we cannot change back.
- Changes that happen again and again, and changes that don’t happen regularly.
- Good changes and bad changes.
- Slow changes and fast changes.
Reversible and Irreversible Changes
Some changes can go back to how they were before. These are called reversible changes.
- For example, when ice melts, it turns into water. We can freeze the water to make ice again.
- Another example is blowing air into a balloon to make it big. We can let the air out to make it small again.
- Adding sugar to water is also reversible. We can heat the water to get the sugar back.
- Folding a paper to make a shape is reversible. We can unfold the paper to make it flat again.
Some changes cannot go back to how they were before. These are called irreversible changes.
- For example, if a balloon bursts, we cannot fix it to make it a balloon again.
- Cutting a paper into pieces is irreversible. We cannot join the pieces to make the paper whole again.
- Baking dough to make chapatti is irreversible. We cannot turn the chapatti back into dough.
Periodic and Non-Periodic Changes
Some changes happen again and again after the same time. These are called periodic changes.
- For example, the sun rises every morning and sets every evening. This happens every day.
- Another example is the changing of seasons like summer, winter, and monsoon. They come every year.
- The moon changes its shape every month. This is also a periodic change.
Some changes do not happen again and again regularly. These are called non-periodic changes.
- For example, rain does not come every day at the same time. This is a non-periodic change.
- An earthquake does not happen regularly. It is also a non-periodic change.
- When a plant wilts and dies, it does not happen at a fixed time. This is a non-periodic change.
Desirable and Undesirable Changes
Some changes are good for us. These are called desirable changes.
- For example, fruits getting ripe is good because we can eat them.
- Burning fuel to run cars is a desirable change because it helps us travel.
- Cooking food is a desirable change because it makes food tasty to eat.
Some changes are not good for us. These are called undesirable changes.
- For example, earthquakes are bad because they can break houses.
- Floods are undesirable because they can destroy homes and things.
- Rusting of iron is bad because it spoils the iron and makes it weak.
Slow and Fast Changes
Some changes take a long time to happen. These are called slow changes.
- For example, milk turning into curd takes a few hours. This is a slow change.
- Another example is rusting of iron. It takes many days or months to happen.
- A child growing tall also takes many years. This is a slow change.
Some changes happen very quickly. These are called fast changes.
- For example, burning paper happens in a few seconds. This is a fast change.
- Breaking a glass also happens quickly. This is a fast change.
Natural and Man-Made Changes
Changes that happen on their own, without human help, are called natural changes.
- Examples of natural changes:
- Rotation of the earth: Earth spins to make day and night.
- Revolution of the earth: Earth moves around the sun to make seasons.
- Change of day into night: Sun goes down, and the moon comes up.
- Formation of coal and petroleum: Rocks and plants turn into coal over many years.
Changes that happen because of human actions are called man-made changes.
- Examples of man-made changes:
- Air pollution: Smoke from cars and factories makes the air dirty.
- Cooking of food: We heat food to make it tasty.
- Making of clothes and furniture: We use cloth and wood to make things.
Physical and Chemical Changes
All changes can be put into two big groups: Physical Changes and Chemical Changes.
Physical Changes
Physical changes are when only the look of a thing changes, like its shape, size, or color. No new thing is made in physical changes, and they can usually go back to how they were.
Physical Properties
Properties of a thing like its size, shape, color, and state are called physical properties.
Characteristics of a Physical Change
- A physical change is temporary and can go back to how it was.
- The thing does not change inside; it stays the same.
- No new thing is made during a physical change.
- In a physical change, only the look changes, like size, shape, color, or state.
- Not much energy is needed for a physical change, and the energy to go back is the same.
- No change in weight happens in a physical change because nothing is added or taken away.
Examples of a Physical Change
- Breaking of a glass plate: When glass breaks, it becomes many pieces, but it is still glass. No new thing is made, so this is a physical change.
- Drying of wet clothes: When wet clothes dry, the water turns into vapor and goes away. The clothes stay the same, so this is a physical change.
- Melting of ice: When ice melts, it turns into water. The ice changes from solid to liquid, but it is still water. No new thing is made, so this is a physical change.
Glowing of an Electric Bulb on the Passage of Electric Current: When you turn on a bulb, electric current makes the filament inside glow and give light. When you turn off the bulb, the filament goes back to normal. No new thing is made in this process. So, glowing of an electric bulb is a physical change.
Melting of Butter, Freezing of Water, Magnetising an Iron Bar by Means of Electricity, Crystallisation of Sugar, and Bending a Glass Tube by Heating Are Some Other Examples of Physical Change.
Let's Perform Some Activities to Understand Physical Changes:
Activity 2.3
- Aim: To show that cutting of paper is a physical change.
- Materials Required: A sheet of paper, a pair of scissors.
- Procedure:
- Take a sheet of paper and cut it into four square pieces.
- Take one square piece and cut it into four smaller square pieces.
- Now try to join all the square pieces by keeping them on a table.
- Observation: You will see that the square pieces cannot be joined back to make the original paper shape.
- Conclusion: This shows that cutting of paper is a physical change because only the shape changes, and no new thing is made.
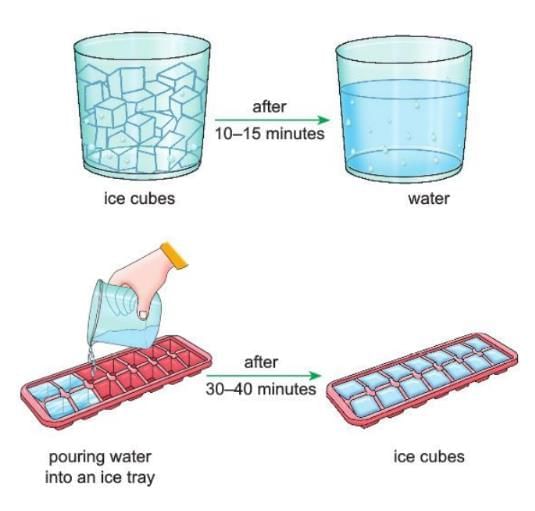
Activity 2.4
- Aim: To show that melting of ice is a physical change.
- Materials Required: A glass tumbler, some ice cubes, an ice tray.
- Procedure:
- Take some ice cubes in a glass tumbler.
- Keep the tumbler at room temperature for 10–15 minutes.
- Now take the water from the tumbler and pour it into an ice tray.
- Keep the ice tray in the freezer for 30–40 minutes.
- Observation: You will see that the ice cubes melt into water. Then, the water freezes back into ice cubes.
- Conclusion: This shows that melting of ice is a physical change because it can go back to ice, and no new thing is made.
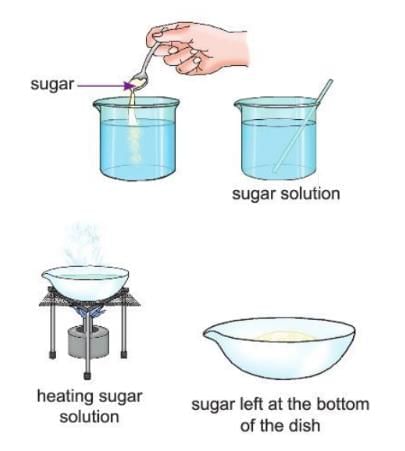
Activity 2.5
- Aim: To show that dissolving sugar in water is a physical change.
- Materials Required: A beaker, some water, some sugar, a spoon, an evaporating dish, a burner.
- Procedure:
- Add two spoonfuls of sugar to water in a beaker and stir.
- Now transfer the sugar solution from the beaker to an evaporating dish.
- Heat the solution until all the water turns into steam.
- Observation: You will see that sugar dissolves in water. Then, when water evaporates, sugar crystals are left behind.
- Conclusion: This shows that dissolving sugar in water is a physical change because sugar can be gotten back, and no new thing is made.
Categorising Physical Changes
Dissolving
- Dissolving is when a solid, liquid, or gas mixes with another thing to make a solution.
- For example, sugar dissolves in water to make a sugar solution.
- This can be reversed by evaporating the water and collecting the sugar back.
Evaporation
- Evaporation is when a liquid turns into a gas at a temperature below its boiling point.
- For example, water in wet clothes turns into vapor when they dry.
- Evaporation is a physical change that is slow, natural, and can be reversed.
- Evaporation depends on things like temperature, surface area, humidity, and air speed.
- Higher temperature makes evaporation faster.
- A bigger surface area makes evaporation faster.
- Lower humidity in the air makes evaporation faster.
- Faster air speed makes evaporation faster.
Boiling
- Boiling is when a liquid turns into a gas when heated to its boiling point.
- For example, water boils and makes bubbles at 100 degrees Celsius.
- Boiling of water is a physical change that is man-made, fast, and can be reversed.
Freezing
- Freezing is when a liquid turns into a solid when it gets cold.
- For example, water turns into ice in the freezer.
- Freezing of water is a physical change that is man-made, reversible, and fast.
Melting
- Melting is when a solid turns into a liquid when heated.
- For example, ice melts into water when it gets warm.
- Melting of ice can be natural or man-made, is fast, and can be reversed.
Condensation
- Condensation is when a gas turns into a liquid when it gets cold.
- For example, water vapor in the air turns into water droplets to form clouds.
- Condensation is a physical change that is natural, reversible, and desirable.
Sublimation
- Sublimation is when a solid turns directly into a gas without becoming a liquid first.
- For example, camphor or naphthalene balls turn into gas to protect clothes from insects.
- Sublimation is a physical change.
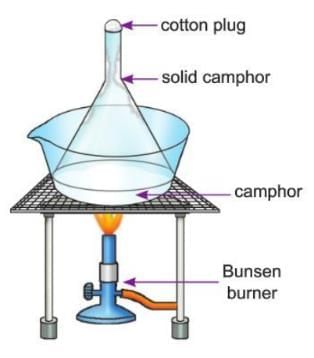
Activity 2.6
- Aim: To show that sublimation of camphor is a physical change.
- Materials Required: A funnel, camphor, cotton wool, an evaporating dish, a burner, a tripod stand, a wire gauze.
- Procedure:
- Take camphor in an evaporating dish.
- Cover the dish by inverting the funnel, as shown in the picture.
- Block the end of the funnel with cotton wool.
- Now heat the camphor using a burner.
- Observation: On heating, camphor turns into gas and rises in the funnel. The gas then cools and turns back into solid camphor on the cooler part of the funnel.
- Conclusion: This shows that sublimation of camphor is a physical change because camphor can go back to its solid form, and no new thing is made.
Chemical Changes
Chemical changes happen when two things mix and make a new thing with different properties. Chemical changes are usually permanent and cannot go back to how they were.
Characteristics of a Chemical Change
- A chemical change is permanent and cannot go back.
- The thing changes inside, and its composition changes.
- One or more new things are made with different properties.
- Energy is given out or taken in during a chemical change.
- A chemical change changes the weight of the thing.
Examples of Chemical Change
- Germination of seeds: A seed grows into a plant and cannot go back to a seed.
- Cooking of food: Food gets heated and becomes new food, like a cake, and cannot go back to raw food.
- Curdling of milk: Milk turns into curd and cannot go back to milk.
- Ripening of fruits: Fruits become sweet and cannot go back to being unripe.
- Rotting of eggs: Eggs go bad and smelly and cannot go back to fresh eggs.
- Burning of fuels: Wood burns and turns into ash, which cannot go back to wood.
Categorising Chemical Changes
Digestion of Food
- When we eat food, it breaks down in our tummy into smaller pieces.
- Big things like proteins, carbs, and fats turn into smaller things with different properties.
- We cannot get the original food back after digestion.
- So, digestion of food is a chemical change.
Rusting of Iron
- When iron is left in wet air, it mixes with oxygen and water to make a brown, rough thing called rust.
- Rust has different properties from iron and cannot go back to iron easily.
- So, rusting of iron is a chemical change.
Cooking of Food
- When we cook food, raw things like grains and vegetables turn into new things.
- Cooked food cannot go back to raw food.
- So, cooking of food is a chemical change.
Burning of Paper
- When paper burns with fire, it turns into ash.
- Ash is a new thing and cannot go back to paper.
- So, burning of paper is a chemical change.
Rotting of Eggs
- When eggs go bad, they become smelly because of a chemical change.
- The sulphur in eggs mixes with hydrogen to make a bad smell.
- This change cannot go back to fresh eggs.
- So, rotting of eggs is a chemical change.
Curdling of Milk
- When we add a starter culture to milk and keep it for some time, it turns into curd.
- Curd cannot go back to milk.
- So, curdling of milk is a chemical change.
Baking a Chapatti
- When we take dough, roll it into a chapatti, and bake it on a griddle, it turns into a baked chapatti.
- The baked chapatti cannot go back to dough.
- So, baking a chapatti is a chemical change.
Fermentation
- Fermentation is when tiny living things break down a thing with heat and carbon dioxide.
- For example, when dough ferments, it becomes soft bread.
- Fermented things cannot go back to how they were.
- So, fermentation is a chemical change.
- Other examples of chemical changes are decomposition of water into hydrogen and oxygen, germination of seeds, and burning of fuels.
Activity 2.7
- Aim: To show that change of color happens during a chemical change.
- Materials Required: A burner, sugar, a pan.
- Procedure:
- Take some sugar in a pan.
- Heat the sugar on the burner.
- Observation: The sugar first melts and turns reddish-brown, then it turns black. The black thing is charcoal.
- Conclusion: This shows that change of color happens in a chemical change because sugar turns into a new thing, charcoal.
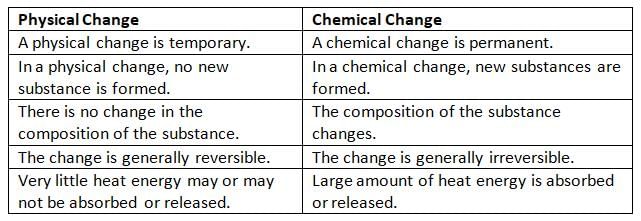
Differences Between Physical Change and Chemical Change
Changes Involving Energy Changes
Most changes involve energy, either taking in energy or giving out energy. Energy is usually in the form of heat.
Changes in Which Energy Is Absorbed:
- Some physical changes take in energy, like melting of ice, boiling of water, and evaporation.
- These changes take in heat because the tiny pieces inside a thing move apart.
- For example, when ice melts, it takes in heat to change from solid to liquid.
- Some chemical changes also take in energy, like photosynthesis, where plants use sunlight to make food.
Changes in Which Energy Is Released:
- Some physical changes give out energy, like freezing of water.
- When water freezes into ice, the tiny pieces come closer, and heat is given out.
- Some chemical changes also give out energy, like combustion and digestion of food.
Points To Remember
- Changes around us can be grouped as reversible and irreversible, periodic and non-periodic, desirable and undesirable, physical and chemical, slow and fast, and natural and man-made changes.
- Changes like dissolving, melting, freezing, boiling, evaporation, and sublimation are physical changes because no new thing is made, and we can get back the original thing.
- Changes like burning, rusting, digestion, cooking, and fermentation are chemical changes because new things are made, and they cannot go back.
- Most changes involve taking in or giving out energy, usually as heat.
- Changes where energy is taken in include melting of ice, boiling of water, and cooking of food.
- Changes where energy is given out include combustion, freezing of water, and digestion of food.
Glossary
- Reversible changes: Changes where we can get back the original thing by doing the opposite action.
- Irreversible changes: Changes where we cannot get back the original thing by doing the opposite action.
- Periodic changes: Changes that happen regularly, like day and night.
- Non-periodic changes: Changes that do not happen regularly, like a sudden rain.
- Desirable changes: Changes that are good for us, like cooking food.
- Undesirable changes: Changes that are not good for us, like food going bad.
- Slow changes: Changes that take a long time, like a plant growing.
- Fast changes: Changes that happen quickly, like breaking glass.
- Natural changes: Changes that happen on their own, like the sun rising.
- Man-made changes: Changes that happen because of human actions, like making a toy.
|
9 videos|46 docs|9 tests
|
FAQs on Physical and Chemical Changes Chapter Notes - Chemistry Class 8 ICSE
| 1. What are the differences between reversible and irreversible changes? |  |
| 2. Can you give examples of periodic and non-periodic changes? |  |
| 3. What are desirable and undesirable changes? |  |
| 4. How do slow and fast changes differ? |  |
| 5. What is the significance of distinguishing between physical and chemical changes? |  |