Year 8 Exam > Year 8 Notes > Year 8 Science IGCSE (Cambridge) > Chapter Notes: Properties of Materials
Properties of Materials Chapter Notes | Year 8 Science IGCSE (Cambridge) PDF Download
| Table of contents |

|
| Dissolving |

|
| Solutions and Solubility |

|
| Planning a Solubility Investigation |

|
| Paper Chromatography |

|
Dissolving
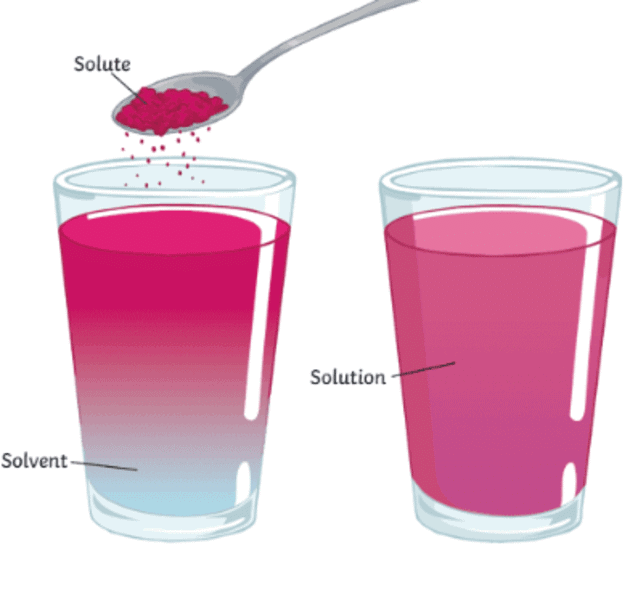
What is a solution?
- Dissolving occurs when a substance, such as a lump of sugar, is placed in a liquid like water, and it gradually seems to disappear.
- The substance that dissolves is called the solute.
- The substance that the solute dissolves into is called the solvent.
- A solution is a mixture of the solute and solvent, e.g., a colourless solution of sugar and water.
- Even though the solute (e.g., sugar) seems to disappear, its particles are still present, spread out among the solvent (e.g., water) particles.
- In the sugar-water example, sugar is the solute, water is the solvent, and the resulting mixture is the colourless solution.
- The process of dissolving involves the solute particles separating and mixing with the solvent particles.
Particle explanation of dissolving
- A sugar crystal is visible because it consists of tightly packed, vibrating groups of particles.
- Water particles, which vibrate and slide past one another, bump into the sugar particles, causing them to separate.
- The movement of water particles helps mix the separated sugar particles with the water particles.
- Eventually, all sugar particles are separated by water particles, becoming too small to be seen individually.
- All solutions are transparent, meaning you can see through them, though they are not necessarily colourless.
- For example, dissolving a coloured salt like copper sulfate in water forms a blue, transparent solution.
- In contrast, liquids like milk are opaque (you cannot see through them) and are not solutions.
Dissolving vs. Melting:
- Dissolving requires two substances: a solute and a solvent (e.g., sugar dissolving in water).
- Melting involves a single substance changing from a solid to a liquid due to heat (e.g., butter melting in a pan).
Examples of dissolving:
- Sugar (solute) in black tea (solvent).
- Instant coffee (solute) in hot water (solvent).
- Nail polish (solute) in nail polish remover (solvent).
Examples of melting:
- Butter in a frying pan.
- Ice cream on a warm day.
- Candle wax as the candle burns.
Mass conservation in dissolving:
- When a solute (e.g., salt) dissolves in a solvent (e.g., water), the solute particles remain in the solution.
- The mass of the solution equals the sum of the mass of the solute and the mass of the solvent.
- Formula: mass of solute + mass of solvent = mass of solution.
- No mass is lost during dissolving; the mass is conserved.
Solutions and Solubility
Solutions
- A solution is formed when a solute dissolves in a solvent.
- Concentrated solution: Contains a large number of solute particles dissolved in the solvent (e.g., a sugar solution with many sugar particles).
- Dilute solution: Contains fewer solute particles dissolved in the solvent (e.g., a sugar solution with fewer sugar particles).
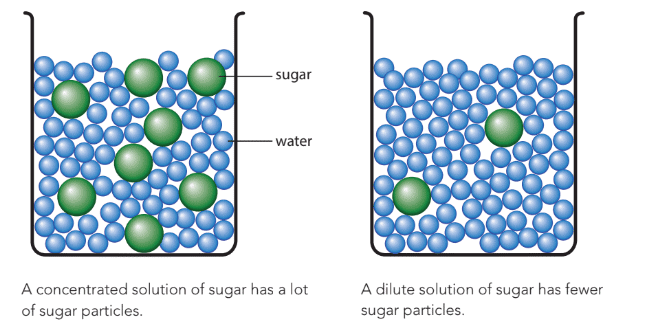
Solubility
- A solid that dissolves in a solvent, such as water, is described as soluble (e.g., sodium chloride and sugar).
- A solid that does not dissolve in a solvent is described as insoluble (e.g., iron filings in water).
- When a soluble solid is continuously added to a solvent, a point is reached where no more solute can dissolve, forming a saturated solution.
- Solubility refers to the amount of solute that can dissolve in a given amount of solvent at a specific temperature.
- Different solutes have varying solubility levels: For example, sodium chloride is highly soluble in water, while lead chloride has low solubility.
Comparing solubility
To compare solubility, measure how much of each solute dissolves in a known amount of solvent under the same conditions (e.g., temperature).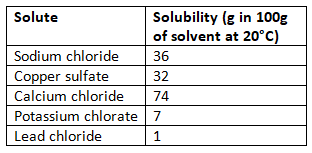
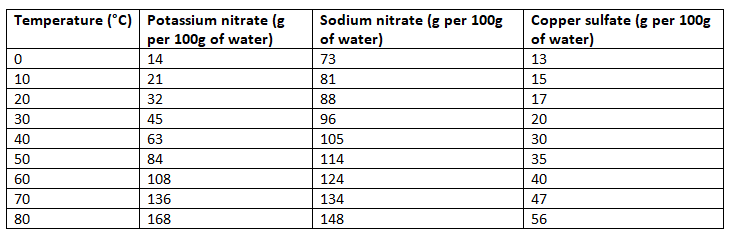
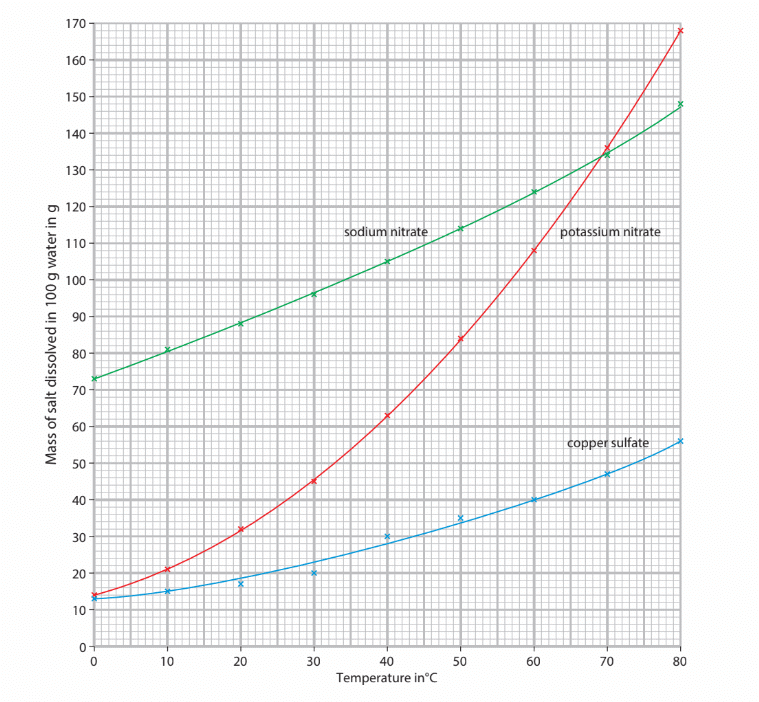
Temperature and Solubility
- Most solutes dissolve more quickly and easily in hot water compared to cold water due to the increased energy of particles.
- With more energy, particles vibrate and move more, facilitating the dissolving process.
- Higher temperatures allow a greater mass of solute to dissolve in the same volume of solvent.
- As temperature increases, the solubility of most solutes also increases.
- Example: In 100 g of water at 20 °C, 204 g of sugar can dissolve, while at 80 °C, 362 g of sugar can dissolve.
Comparing the solubility of different salts
Other Solvents
- Water is not the only solvent; other liquids can also act as solvents for specific solutes.
- Some substances that are insoluble in water can dissolve in alternative solvents.
- Example: Oil paint, which is insoluble in water, dissolves in methanol (methylated spirits), used to clean paint brushes.
- Example: Nail polish, which does not dissolve in water, dissolves in nail polish remover containing propanone (acetone) as the solvent.
Planning a Solubility Investigation
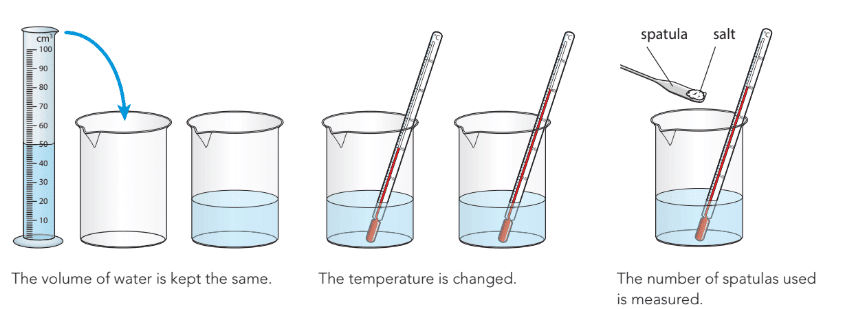
Dissolving Salt in Water
- An investigation can be designed to study how temperature affects the amount of salt (sodium chloride) that dissolves in water.
- Variables in the investigation:
- Independent variable: The variable that is changed, e.g., the temperature of the water.
- Dependent variable: The variable that is measured, e.g., the amount of salt (number of spatulas) that dissolves.
- Control variables: Variables that are kept constant to ensure a fair test, e.g., the volume of water.
- When plotting results:
- The independent variable (e.g., temperature) is plotted on the horizontal axis.
- The dependent variable (e.g., amount of salt dissolved) is plotted on the vertical axis.
Paper Chromatography
- Paper chromatography is a technique used to separate dissolved substances in a mixture.
- It involves using a solvent to move substances along a piece of paper, separating them based on their solubility and interaction with the paper.
- The result of paper chromatography is called a chromatogram, which shows the separated substances as distinct spots or bands.
- Solvent front: The furthest point reached by the solvent on the chromatography paper.
- Permanent: Refers to substances (e.g., inks or dyes) that do not break down or change during the chromatography process.
Colours in Ink
- Black ink appears as a single colour but is actually a mixture of different coloured inks.
- Paper chromatography is a technique used to separate the different coloured inks within a mixture, such as black ink.
- Special paper, similar to filter paper, is used in paper chromatography.

Process of paper chromatography
- A small drop of ink (e.g., black ink) is placed on the chromatography paper.
- The paper is placed in a beaker with a solvent (e.g., water) so that the solvent soaks up into the paper.
- As the solvent moves up the paper, it carries the ink particles along, separating the different coloured inks that make up the original ink.
- The resulting image on the paper, showing the separated colours, is called a chromatogram.

Mechanism of separation
- The solvent (e.g., water) dissolves the ink particles.
- As the solvent moves up the paper, it carries the ink particles different distances based on their solubility.
- Ink particles with higher solubility are carried further up the paper, while less soluble particles are left behind sooner.
- Different inks (e.g., green, black, red) produce distinct chromatograms, revealing the various coloured components within each ink.
- Some inks, such as those in permanent marker pens, are not soluble in water and require a different solvent, such as alcohol, to separate their components.
Applications of chromatography
- Scientists use chromatography to study dyes in food to determine if they are pure substances or mixtures.
- A pure substance produces a single spot on the chromatogram, while a mixture produces multiple spots.
- Identifying the composition of food dyes is important to ensure they are safe and do not contain harmful substances that could be toxic or cause allergic reactions.
- Public health scientists use chromatography to check the safety of colourings in products like hair dye or pen ink by comparing chromatograms of the product with those of permitted dyes.
The document Properties of Materials Chapter Notes | Year 8 Science IGCSE (Cambridge) is a part of the Year 8 Course Year 8 Science IGCSE (Cambridge).
All you need of Year 8 at this link: Year 8
|
3 videos|56 docs|9 tests
|
FAQs on Properties of Materials Chapter Notes - Year 8 Science IGCSE (Cambridge)
| 1. What happens when salt is dissolved in water? |  |
Ans.When salt dissolves in water, it breaks down into its constituent ions, sodium (Na+) and chloride (Cl-). This process is called dissociation, and it occurs because water molecules surround the salt ions, pulling them apart and dispersing them throughout the solution.
| 2. How can you separate dissolved salt from water? |  |
Ans.Dissolved salt can be separated from water through evaporation. By heating the saltwater solution, the water will gradually evaporate, leaving the salt behind. This method is commonly used in salt production.
| 3. Why do different colors appear in ink when mixed with water? |  |
Ans.Different colors appear in ink when mixed with water due to the various pigments and dyes used in the ink. Each pigment has a unique solubility in water, causing them to separate and create a spectrum of colors when they dissolve.
| 4. What tools can I use to observe the properties of materials in a scientific manner? |  |
Ans.To observe the properties of materials scientifically, you can use tools such as a microscope for examining small structures, a balance for measuring mass, and a thermometer for measuring temperature. These tools help provide accurate measurements and observations.
| 5. How can I check my understanding of the properties of materials? |  |
Ans.You can check your understanding of the properties of materials by completing practice quizzes, conducting experiments to observe material behaviors, and reviewing summary checklists that highlight key concepts and definitions related to the topic.
Related Searches















