Reactions and Equations Chapter Notes | Technical Science for Grade 10 PDF Download
| Table of contents |

|
| Compounds Can Decompose to Elements |

|
| Some Substances Form Ions in Water, But Others Do Not |

|
| Naming Compounds |

|
| Points to Remember |

|
| Difficult Words |

|
| Summary |

|
Compounds Can Decompose to Elements
This section explores how compounds can break down into their constituent elements through chemical reactions, using historical experiments to illustrate the process and introducing the concept of balancing chemical equations.
Decomposition of Compounds
- Definition: Decomposition is a chemical reaction where a compound breaks down into its elements or simpler substances.
- Explanation: Compounds are made of two or more elements bonded in fixed ratios. When energy (e.g., heat or electricity) is applied, these bonds can break, releasing the elements.
- Historical Example: In 1774, Joseph Priestley heated an orange powder called mercuric oxide (HgO) using sunlight focused through magnifying glasses. The powder darkened, produced liquid mercury droplets, and released a gas later identified as oxygen.
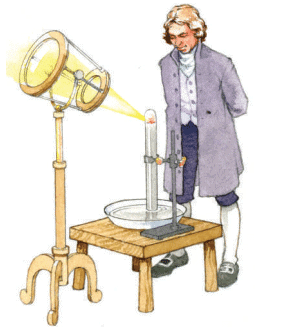
- Significance: Priestley’s experiment showed that mercuric oxide, a compound, decomposes into its elements, mercury (Hg) and oxygen (O₂). This helped establish oxygen’s role in air and its necessity for life.
Structure of Mercuric Oxide
- Explanation: Mercuric oxide (HgO) is a powder where each grain consists of billions of mercury and oxygen atoms arranged in a regular pattern called a lattice.
- Lattice: A lattice is a three-dimensional, repeating structure of atoms or ions in a solid compound.
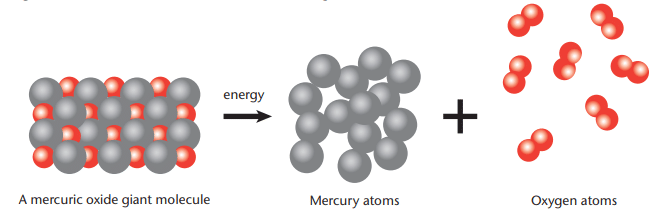
- Decomposition Process: When heated, the energy breaks the bonds in the HgO lattice. Mercury atoms join to form large molecules, visible as liquid mercury droplets. Oxygen atoms pair up to form oxygen molecules (O₂), released as a gas.
Chemical Reactions
- Definition: A chemical reaction occurs when atoms that were bonded break apart and form new bonds with other atoms, creating new substances.
- Example: In Priestley’s experiment, mercuric oxide (HgO) reacts to form mercury (Hg) and oxygen (O₂).
- Word Equation: Mercuric oxide breaks up to form mercury and oxygen.
Picture Models
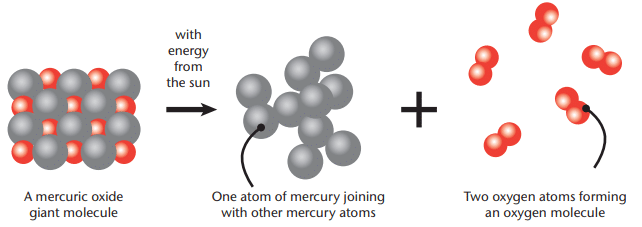
Explanation: A picture model is a diagram showing the atoms and molecules before and after a reaction. It helps visualize how atoms rearrange.Example: For mercuric oxide decomposition, the picture model shows a giant HgO molecule (a lattice) breaking into mercury atoms and oxygen molecules (O₂).
- Left Side: 10 mercury atoms and 10 oxygen atoms in HgO.
- Right Side: 10 mercury atoms and 5 oxygen molecules (each O₂ has 2 oxygen atoms, totaling 10 oxygen atoms).
Purpose: The model ensures the number of each type of atom is the same before and after the reaction, illustrating conservation of matter.
Conservation of Matter
- Definition: In a chemical reaction, matter is conserved, meaning no atoms are created or destroyed. The number of each type of atom remains the same before and after the reaction.
- Explanation: This principle allows chemists to write chemical equations where the number of atoms on the left (reactants) equals the number on the right (products).
- Example: In HgO decomposition, 10 mercury atoms and 10 oxygen atoms on the left equal 10 mercury atoms and 10 oxygen atoms (in 5 O₂ molecules) on the right.
Balancing Chemical Equations
Definition: A chemical equation is a symbolic representation of a chemical reaction, showing reactants (starting substances) and products (resulting substances).Balancing: An equation is balanced when the number of each type of atom is the same on both sides of the arrow.
Example (Mercuric Oxide):
- Unsimplified Equation: 10 HgO → 10 Hg + 5 O₂ (10 mercury atoms, 10 oxygen atoms on both sides).
- Simplified Equation: 2 HgO → 2 Hg + O₂ (2 mercury atoms, 2 oxygen atoms on both sides).
- Ratio: For every 2 HgO molecules, 2 Hg atoms and 1 O₂ molecule are produced.
Symbols: Hg is mercury, O is oxygen, and the arrow (→) indicates the reaction direction from reactants to products.
Decomposition of Water
Historical Context: Alchemists once thought water was an element, but in 1799, Alessandro Volta’s battery inspired experiments proving water is a compound.Experiment: Scientists used a battery to pass electricity through water, producing bubbles of hydrogen (H₂) at the negative electrode and oxygen (O₂) at the positive electrode.
Observation: Twice as much hydrogen gas was produced compared to oxygen gas (e.g., 2 billion hydrogen molecules vs. 1 billion oxygen molecules).
Inference: Water (H₂O) contains hydrogen and oxygen in a 2:1 atom ratio (two hydrogen atoms per oxygen atom).
Word Equation: Water, with energy from a battery, breaks down into hydrogen and oxygen.
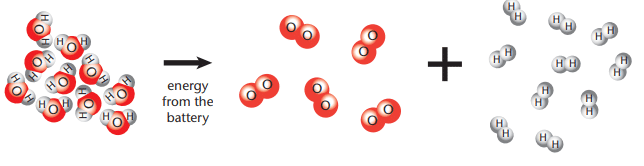
Picture Model: Shows 2 H₂O molecules decomposing into 2 H₂ molecules and 1 O₂ molecule.
Chemical Equation: 2 H₂O → 2 H₂ + O₂ (4 hydrogen atoms, 2 oxygen atoms on both sides).
A picture model of water decomposing into hydrogen and oxygen below:
Forming Compounds from Elements
Explanation: Elements can react to form compounds, the reverse of decomposition.Example: Magnesium (Mg) reacts with oxygen (O₂) in the air when heated, producing a white powder, magnesium oxide (MgO).
- Reactants: Magnesium and oxygen.
- Product: Magnesium oxide, a compound with small white crystals.
Word Equation: Magnesium and oxygen react to form magnesium oxide.=
Chemical Equation: 2 Mg + O₂ → 2 MgO (2 magnesium atoms, 2 oxygen atoms on both sides).
Picture Model: Shows magnesium atoms and oxygen molecules combining to form MgO molecules.
Other Decomposition Reactions
Examples:
Acetylene and Oxygen: Used in flame-cutting steel, acetylene (C₂H₂) and oxygen (O₂) react with heat to form carbon dioxide (CO₂) and water (H₂O).
- Unbalanced Equation: C₂H₂ + O₂ → CO₂ + H₂O.
- Balanced Equation: 2 C₂H₂ + 5 O₂ → 4 CO₂ + 2 H₂O.
Nitrogen Dioxide: In catalytic converters, nitrogen dioxide (NO₂) decomposes with heat into oxygen (O₂) and nitrogen (N₂).
- Unbalanced Equation: NO₂ → O₂ + N₂.
- Balanced Equation: 2 NO₂ → O₂ + N₂.
Iron Oxide Smelting: Indigenous iron-workers used carbon (C) to reduce iron oxide (Fe₂O₃) to iron (Fe) and carbon dioxide (CO₂).
- Unbalanced Equation: Fe₂O₃ + C → Fe + CO₂.
- Balanced Equation: 2 Fe₂O₃ + 3 C → 4 Fe + 3 CO₂.
Some Substances Form Ions in Water, But Others Do Not
This section explains why some substances form ions when dissolved in water while others do not, focusing on water’s polar nature and its interaction with compounds like sugar and salt.
Properties of Water (Macro-Scale)
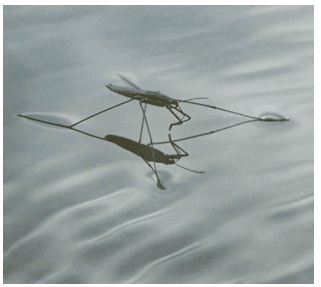
Explanation: Water has unique properties that make it essential for life.
Examples:
- In humans, water carries dissolved substances to organs.
- In plants, water clings to tiny tubes, rising to leaves and carrying nutrients from soil.
Surface Tension: Water’s surface acts like an elastic skin due to a force called surface tension, allowing insects like pond-skaters to walk on it or a steel needle to float.
Demonstration: When two small water drops on a smooth surface touch, they merge into one round drop, showing water molecules’ attraction.
Water on the Nano-Scale
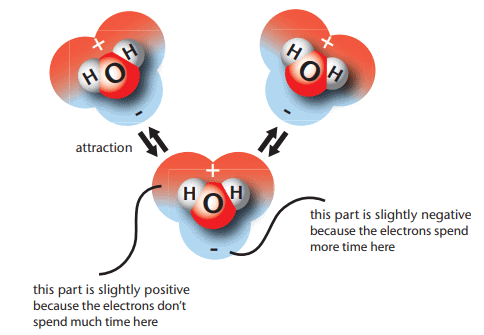
Water Molecule Structure: A water molecule (H₂O) consists of two hydrogen atoms bonded to one oxygen atom.Polarity: Water is a polar molecule, meaning it has a positive and a negative end.
- Reason: Oxygen, on the right side of the periodic table, pulls shared valence electrons more strongly than hydrogen, on the left side.
- Result: The oxygen end is slightly negative (due to electron attraction), and the hydrogen end is slightly positive.
Hydrogen Bonding: The positive hydrogen end of one water molecule attracts the negative oxygen end of another, creating hydrogen bonds.
Effect: Hydrogen bonds cause water molecules to pull sideways on the surface, forming the “skin” (surface tension).
Dissolving Substances: Hydrogen bonds allow water to pull on slightly positive or negative parts of other molecules, helping them dissolve.
Dissolving Sugar in Water
Macro-Scale: When sugar is stirred into water, it disappears, forming an even mixture called a solution (homogeneous mixture).Nano-Scale:
- Process: Water molecules attract slightly positive hydrogen atoms in sugar molecules, pulling them out of the sugar crystal.
- Result: Sugar molecules spread among water molecules but remain intact, not breaking into smaller parts.
 Sugar dissolves in water.
Sugar dissolves in water.
Key Point: Sugar dissolves as whole molecules, not as ions.
Dissolving Salt in Water
Macro-Scale: Salt (NaCl) dissolves in water, forming a solution, but the process differs from sugar.Nano-Scale:
- Salt Structure: NaCl forms a crystal lattice of sodium (Na) and chlorine (Cl) atoms.
- Electron Pulling: Chlorine, on the right side of the periodic table, pulls the shared electron from sodium more strongly.
- Water’s Role: Water’s positive hydrogen ends tug at chlorine atoms, and negative oxygen ends tug at sodium atoms.
Ion Formation:
- When a sodium atom is pulled from the crystal, chlorine keeps the shared electron, leaving sodium with a positive charge (Na⁺, a cation).
- When chlorine is pulled out, it retains the extra electron, becoming negatively charged (Cl⁻, an anion).

A salt crystal
Key Point: Salt dissolves by breaking into positive and negative ions, unlike sugar.
Ions and Conductivity
Definition: Ions are atoms or molecules that have gained or lost electrons, giving them a positive (cations) or negative (anions) charge.
Examples:
- Sodium chloride (NaCl) forms Na⁺ and Cl⁻ ions.
- Copper sulfate (CuSO₄) forms Cu²⁺ and SO₄²⁻ ions.
Conductivity: Solutions with ions conduct electricity because ions move and carry electric charge.
- High Conductivity: Solutions with many ions (e.g., concentrated salt or copper sulfate solutions) conduct well.
- Low Conductivity: Solutions with few ions (e.g., tap water) conduct poorly.
- No Conductivity: Solutions with no ions (e.g., sugar solution, pure water) do not conduct.
Measurement: Conductivity is measured in Siemens per meter (S/m) or Siemens per centimeter (S/cm), using a tester with electrodes 1 cm apart.
Industrial Applications
Explanation: In industries like power stations, water for boilers must have very low ion content to prevent pipe blockages from dissolved substances.
Conductivity Testing: Chemists use conductivity testers to check water purity.
- Pure Water: Almost no ions, no conductivity.
- Tap/Borehole Water: Some dissolved substances, slight conductivity.
- High Conductivity: Indicates too many dissolved compounds, a warning for industrial use.
Naming Compounds
This section covers the rules for naming chemical compounds, focusing on the use of the periodic table, valency, and the table of cations and anions.
Importance of Naming Compounds
- Explanation: With millions of substances, chemists need standardized names to communicate clearly.
- Historical Context: Early chemists used informal names (e.g., Epsom salts for magnesium sulfate, Condy’s crystals for potassium permanganate). Modern naming follows strict rules.
Naming Rules
Rule: The element farther to the left in the periodic table is named first, followed by the element to the right.
Examples:
- Sodium (Na, left) and chlorine (Cl, right) form sodium chloride (NaCl), not “chlorine sodium.”
- Potassium (K, left) and iodine (I, right) form potassium iodide (KI).
- Hydrogen (H, left) and sulfur (S, right) form hydrogen sulfide (H₂S).
- Carbon (C, left) and oxygen (O, right) form carbon dioxide (CO₂).
Prefixes:
- Di-: Indicates two atoms of an element (e.g., carbon dioxide, CO₂).
- Tri-: Indicates three atoms (e.g., sulfur trioxide, SO₃).
Exceptions: Some names omit prefixes for simplicity (e.g., calcium chloride, CaCl₂, instead of calcium dichloride; ferric chloride, FeCl₃, instead of iron trichloride).
Cations and Anions
Definition:
Cations: Positive ions, usually metal atoms (e.g., Na⁺, Cu²⁺).
Anions: Negative ions, either non-metal atoms (e.g., Cl⁻) or groups of atoms called radicals (e.g., SO₄²⁻, CO₃²⁻).
Table of Cations and Anions: Lists common ions with their charges (e.g., 1+, 2+, 1-, 2-).
Examples:
Cations: Sodium (Na⁺), calcium (Ca²⁺), iron(III) (Fe³⁺).
Anions: Chloride (Cl⁻), sulfate (SO₄²⁻), hydrogen carbonate (HCO₃⁻).
Radicals: Groups of atoms acting as a single anion (e.g., carbonate, CO₃²⁻).
Stock Notation
Explanation: For elements with multiple possible valencies (number of bonds or charges), Roman numerals indicate the charge in the compound.
Examples:
- Copper(I) chloride (CuCl): Copper has a 1+ charge.
- Copper(II) chloride (CuCl₂): Copper has a 2+ charge.
- Iron(II) oxide (FeO): Iron has a 2+ charge.
- Iron(III) oxide (Fe₂O₃): Iron has a 3+ charge.
Examples of Compound Names and Formulas
Given Names, Find Formulas:
- Magnesium oxide: MgO
- Carbon dioxide: CO₂
- Sodium chloride: NaCl
- Sulfur trioxide: SO₃
- Copper(II) sulfate: CuSO₄
Given Formulas, Find Names:
- MgO: Magnesium oxide
- CO₂: Carbon dioxide
- NaCl: Sodium chloride
- Fe₂O₃: Iron(III) oxide
- CuSO₄: Copper(II) sulfate
Points to Remember
- Compounds can decompose into their elements through chemical reactions (e.g., mercuric oxide → mercury + oxygen).
- Elements can react to form compounds (e.g., magnesium + oxygen → magnesium oxide).
- Chemical equations represent reactions and must be balanced, with equal numbers of each atom type before and after the reaction.
- Matter is conserved in chemical reactions; no atoms are created or destroyed.
- Water is a polar molecule with a positive hydrogen end and a negative oxygen end, enabling hydrogen bonding and dissolution of substances.
- Some compounds (e.g., sugar) dissolve as whole molecules, while others (e.g., salt, copper sulfate) break into positive (cations) and negative (anions) ions.
- Solutions with ions conduct electricity; conductivity depends on the number of ions per liter.
- In industry, low-conductivity water is critical for boilers to prevent pipe blockages.
- Compounds are named with the leftmost periodic table element first (e.g., sodium chloride, not chlorine sodium).
- Stock notation uses Roman numerals for elements with multiple valencies (e.g., copper(II) chloride, CuCl₂).
Difficult Words
- Decomposition: A chemical reaction where a compound breaks down into its elements or simpler substances.
- Chemical Reaction: A process where atoms break old bonds and form new bonds, creating new substances.
- Lattice: A regular, three-dimensional arrangement of atoms or ions in a solid compound.
- Picture Model: A diagram showing atoms and molecules before and after a reaction to visualize atom rearrangement.
- Conservation of Matter: The principle that no atoms are created or destroyed in a chemical reaction.
- Chemical Equation: A symbolic representation of a reaction, showing reactants and products with an arrow.
- Reactants: Starting substances in a chemical reaction.
- Products: Substances formed in a chemical reaction.
- Polar Molecule: A molecule with a positive and negative end, like water (H₂O).
- Hydrogen Bonding: Attraction between the positive end of one molecule and the negative end of another, common in water.
- Solution: An even (homogeneous) mixture of substances, like sugar or salt dissolved in water.
- Ion: An atom or molecule with a positive (cation) or negative (anion) charge due to gaining or losing electrons.
- Cation: A positively charged ion, usually a metal (e.g., Na⁺).
- Anion: A negatively charged ion, often a non-metal or radical (e.g., Cl⁻, SO₄²⁻).
- Conductivity: The ability of a solution to conduct electricity, depending on the presence of ions.
- Stock Notation: Using Roman numerals to indicate the charge of an element with multiple valencies (e.g., iron(III)).
- Radical: A group of atoms acting as a single anion (e.g., carbonate, CO₃²⁻).
Summary
This chapter explores chemical reactions and equations, focusing on how compounds decompose into elements, how substances behave in water, and how compounds are named. Compounds like mercuric oxide and water can break down into their elements (e.g., mercury and oxygen, or hydrogen and oxygen) through reactions, which are represented by balanced chemical equations ensuring matter conservation. Elements can also combine to form compounds, such as magnesium and oxygen forming magnesium oxide. Water’s polar nature, with positive and negative ends, enables it to dissolve substances. Some compounds, like sugar, dissolve as whole molecules, while others, like salt and copper sulfate, form ions (cations and anions), making their solutions conductive. Conductivity testing is vital in industries to ensure water purity. Compounds are named using periodic table positions, with the leftmost element first, and Stock notation for variable valencies. These concepts help explain how atoms combine and react to form new substances.
FAQs on Reactions and Equations Chapter Notes - Technical Science for Grade 10
| 1. What are the main types of compounds that can decompose into elements? |  |
| 2. Why do some substances form ions in water while others do not? |  |
| 3. How do you name compounds correctly? |  |
| 4. What are some difficult words related to reactions and equations? |  |
| 5. What is the importance of understanding reactions and equations in chemistry? |  |














