States of Matter Chapter Notes | Science for Grade 6 PDF Download
Introduction
The chapter "States of Matter" helps us understand how different materials can exist as solids, liquids, or gases. It explains why some things, like gallium, can change from a solid to a liquid when warmed, like in your hand. We will learn about how energy affects the way particles move and how they are arranged in different states. This chapter also talks about why different substances change states at different temperatures and how energy plays a role in these changes.
What happens to temperature during a change of state?
- When a solid, like gallium, is warmed, it takes in heat from your hand, which is warmer than room temperature.
- Adding energy to a solid increases the speed of its particles, making them move faster.
- This increase in speed is due to an increase in kinetic energy, which is the energy of moving particles.
- When a solid gets enough energy, it can change into a liquid, like ice melting into water.
Changes Between Solids and Liquids
- When a solid, like ice, reaches a certain temperature (0°C for ice), it starts to melt and becomes a liquid.
- During melting, the temperature stays the same until all the solid has turned into a liquid.
- The temperature at which a solid turns into a liquid is called the melting point.
- The temperature at which a liquid turns into a solid is called the freezing point.
- For any substance, the melting point and freezing point are the same temperature.
- When a liquid changes to a solid, the temperature also stays constant until the change is complete.
Changes Between Gases and Liquids
- When a gas cools down enough, it turns into a liquid. This process is called condensation.
- The opposite process, where a liquid turns into a gas, is called vaporization.
- Vaporization happens in two ways: evaporation and boiling.
- Evaporation is when a liquid turns into a gas at its surface, like water slowly disappearing from a glass at room temperature.
- Boiling is when a liquid turns into a gas inside the liquid, forming bubbles, and happens at a specific temperature called the boiling point.
- The boiling point and condensation point are the same for a substance, and the temperature stays constant during these changes until the process is complete.
Difference Between Evaporation and Boiling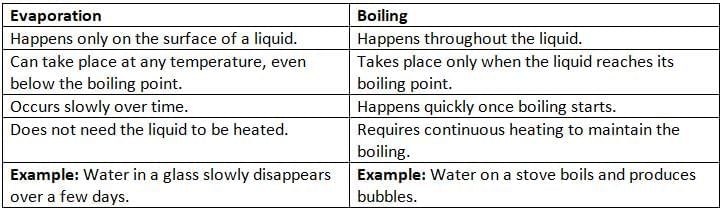
Did You Know?
The water cycle is powered by changes in the states of matter! Water from the ground turns into a gas and rises into the air. This gas, called water vapor, cools down and changes back into tiny water droplets, forming clouds — a process known as condensation. Sometimes at night, this condensation creates dew on grass and other cool surfaces.
What happens to particles and energy during a change of state?
Particle Arrangement
- When energy is continuously added to a substance, there comes a point where the particles cannot move any faster unless the substance changes to a different state of matter.
- In gases, particles move quickly and are spread far apart from one another.
- In liquids, particles are close together but still have the ability to slide past each other.
- In solids, the particles are tightly packed and held in a fixed, rigid structure.
- The reason why solids, liquids, and gases have different shapes is due to the strength of the attractions between their particles in each state.
Particle Attraction
- When energy is added to a substance and the particles can no longer move faster in their current state, the extra energy is used to break the attraction between the particles, leading to a change in state.
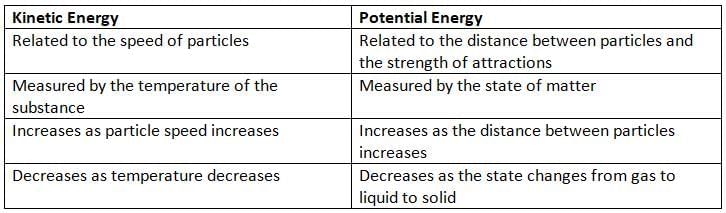
- This added energy increases the potential energy of the particles.
- Potential energy is stored energy that comes from the interactions between particles or objects.
- As the distance between particles increases, their potential energy also increases.
- On the other hand, when the distance between particles decreases, their potential energy decreases.
- Particles that are farther apart have more potential energy.
- The amount of potential energy, which depends on how far apart the particles are in a particular state of matter, adds to the total energy of the substance.
Difference Between Kinetic and Potential Energy
Heating Curves
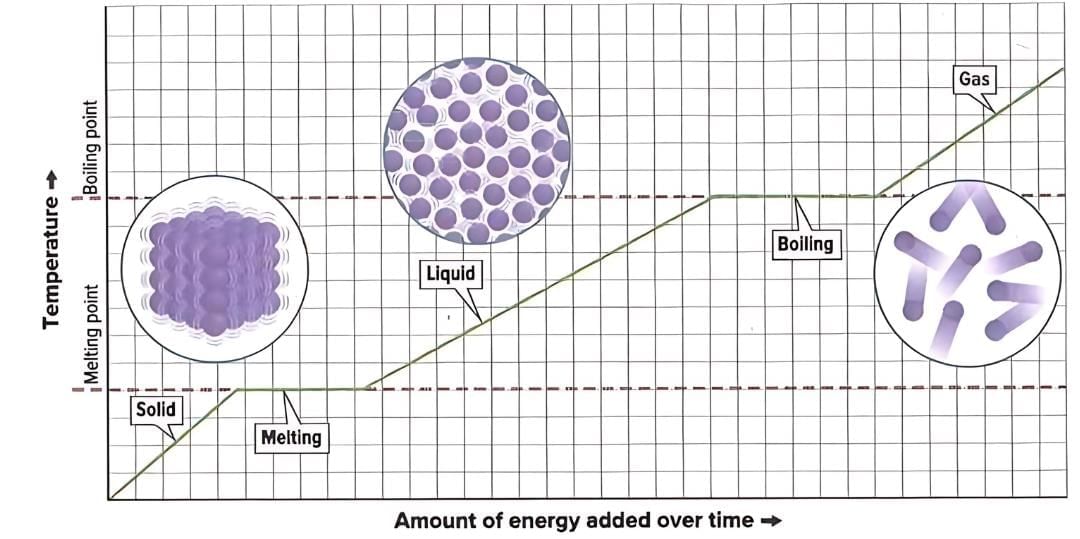
- A heating curve is a graph that shows how a substance’s temperature changes as it gains energy.
- When a substance is not changing state, adding energy increases its temperature and the kinetic energy of its particles, making them move faster.
- During a state change, like melting or boiling, the temperature stays the same even though energy is being added.
- During state changes, the energy increases the potential energy of particles, which increases the distance between them.
How do the melting and boiling points of different substances compare?
- Each substance has its own unique melting and boiling points.
- For example, ice melts at 0°C and water boils at 100°C, but other substances like gallium melt at different temperatures.
- The melting and boiling points depend on how strongly the particles in a substance are attracted to each other.
- Substances with stronger particle attractions need more energy to change state, so they have higher melting and boiling points.
- Different substances are in different states at the same temperature because of the differences in particle attractions.
What factors determine the total energy of a substance?
- The total energy of a substance depends on several factors.
- Kinetic energy: This is based on how fast the particles are moving, which is measured by temperature.
- Potential energy: This depends on how the particles are arranged and their state of matter.
- Mass: The total number of particles in a substance affects its energy. More mass means more particles and more energy.
- Type of matter: Different substances have different particle attractions, which affect how much energy they have.
- Thermal energy is the total energy of a substance, including both kinetic and potential energy, and depends on the number of particles, state of matter, and temperature.
- Thermal energy is different from temperature because temperature only measures the average kinetic energy of particles.
- For example, liquid metal and solid metal at the same temperature have different thermal energies because the liquid has more potential energy due to its particle arrangement.
A Closer Look: Fractional Distillation
- Fractional distillation is a process used to separate gases in the air, like nitrogen and oxygen, based on their boiling points.
- Air is made up of 78% nitrogen, 21% oxygen, and 1% other gases, like argon.
- The process starts by filtering out soot and dirt from the air.
- The air is cooled in a heat exchanger, releasing heat to a cooler fluid.
- The cooled air is compressed and passed through a nozzle into a larger chamber, where it cools further and turns into a liquid.
- The liquid air is warmed to the boiling point of nitrogen (-195.8°C), causing most of the nitrogen to turn into a gas, with a small amount of oxygen.
- The remaining liquid oxygen and nitrogen are collected and distilled again at a higher temperature to separate them further.
- After several rounds of distillation, the separated nitrogen and oxygen are liquefied again and stored in special insulated containers.
- Argon, which makes up part of the 1% of other gases, is hard to separate from liquid oxygen because its boiling point is close to oxygen’s.
|
124 docs|8 tests
|
FAQs on States of Matter Chapter Notes - Science for Grade 6
| 1. What happens to temperature during a change of state? |  |
| 2. How do the melting and boiling points of different substances compare? |  |
| 3. What happens to particles and energy during a change of state? |  |
| 4. What factors determine the total energy of a substance? |  |
| 5. What is fractional distillation, and how is it used? |  |




















