Class 3 Exam > Class 3 Notes > Science for Class 3 > Chapter Notes: States of Matter
States of Matter Class 3 Notes Science
| Table of contents |

|
| States of Matter |

|
| Three State of Matter |

|
| Change of States |

|
| Conclusion |

|
States of Matter

- Everything we see around us, like tables, chairs, water, and air, takes up space and has weight. These things are called matter. Matter is everywhere.
- Scientists say that matter can be solid like a table, liquid like water, or a gas like air.
- Matter is made of tiny parts called molecules, and these molecules are put together in different ways in different things.
Three State of Matter
1. Solids
- Solid is a state of matter that has a definite shape and volume.
- In solids, molecules are closely packed. That is why solids are hard.
- Solids do not change their shape when moved from one container to another container, i.e., they have a definite shape and a definite volume.
- For example, chair, dice, cup, book, wood, etc.

2. Liquid
- Liquid is a state of matter that does not have a definite shape and flows easily.
- The molecules that make up a liquid are loosely packed than those in solids and are able to move freely.
- Liquids take the shape of the container in which they are kept and have a fixed volume.
- For example, water, oil, milk, juice, etc.

Gases
- Like liquids, gases also do not have a definite shape.
- The molecules that make up gases are more loosely packed than liquids.
- They are able to move randomly.
- Like liquids, gases take the shape of the container they are kept in.
- They do not have a fixed volume.
- Air is a mixture of many gases.
- Examples of gases are oxygen, carbon dioxide, hydrogen, nitrogen, etc.
Question for Chapter Notes: States of MatterTry yourself: What state of matter has a definite shape and volume?View Solution
Change of States
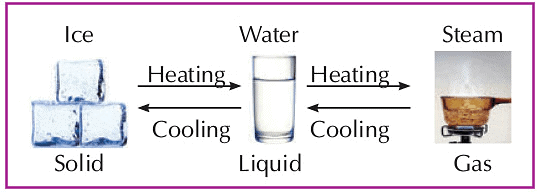
- States of matter can be changed from one form to another.
- A solid can change into a liquid on heating.
- On heating, a liquid becomes a gas.
- On cooling down, a gas becomes a liquid.
- When we freeze a liquid, it becomes solid.
- For example, on heating, ice changes into water (liquid), which on further heating, changes into vapours.
Conclusion
Matter around us can exist in three different states: solid, liquid, and gas. Each state has unique properties based on how its molecules are arranged and move. Changes in temperature can cause matter to switch from one state to another, like ice melting into water or water evaporating into vapor. Understanding these states and changes helps us see how things around us work and interact in everyday life.
Question for Chapter Notes: States of MatterTry yourself: Which state of matter is formed when a gas is cooled down?View Solution
The document States of Matter Class 3 Notes Science is a part of the Class 3 Course Science for Class 3.
All you need of Class 3 at this link: Class 3
|
19 videos|48 docs|30 tests
|
FAQs on States of Matter Class 3 Notes Science
| 1. What are the three main states of matter? |  |
Ans.The three main states of matter are solid, liquid, and gas. Solids have a fixed shape and volume, liquids have a fixed volume but take the shape of their container, and gases have neither a fixed shape nor a fixed volume.
| 2. How do the particles in solids differ from those in liquids and gases? |  |
Ans.In solids, the particles are closely packed together and vibrate in place, which keeps the solid's shape. In liquids, the particles are still close but can slide past each other, allowing the liquid to flow. In gases, the particles are far apart and move freely, filling the entire space available to them.
| 3. What happens to matter when it changes states? |  |
Ans.When matter changes states, the arrangement and energy of the particles change. For example, when ice (solid) melts into water (liquid), the particles gain energy and move more freely. Similarly, when water boils and turns into steam (gas), the particles gain even more energy and spread apart.
| 4. Can you give an example of a state change? |  |
Ans.An example of a state change is when water freezes to become ice. As the temperature drops, the energy of the water particles decreases, causing them to come closer together and form a solid structure, which is ice.
| 5. Why is it important to understand the states of matter? |  |
Ans.Understanding the states of matter is important because it helps us comprehend how different materials behave in various conditions. This knowledge is essential in everyday life, science, and industry, influencing everything from cooking to engineering.
|
Explore Courses for Class 3 exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches

















