Sterilization and Disinfection Chapter Notes | Microbiology - NEET PG PDF Download
| Table of contents |

|
| Definitions |

|
| Physical Methods of Sterilization/Disinfection |

|
| Chemical Agents of Sterilization/Disinfection |

|
| Testing of Disinfectants |

|
Definitions
- Sterilization:. process that eradicates all living microorganisms, including resilient spores, achieving a minimum reduction of 106 log colony-forming units (CFU).
- Disinfection: This process targets and eliminates most pathogenic organisms, excluding bacterial spores, with a CFU reduction of at least 103 log.
- Asepsis: The application of chemical agents, known as antiseptics, to skin surfaces to inhibit or destroy pathogenic microorganisms and some natural skin bacteria.
- Decontamination (or Sanitization): Reducing pathogenic microbes to a safe level, allowing items to be handled without protective gear, with a minimum 1 log CFU reduction of microorganisms, excluding spores.
Factors Affecting the Effectiveness of Sterilants and Disinfectants
Several factors influence how effective a sterilant or disinfectant is:
- Organism Load: The time required to eliminate microbes increases with larger populations.
- Nature of Organisms: The effectiveness of disinfectants varies based on the type of organisms present.
- Concentration and Temperature: The physical factors of concentration and temperature are crucial for effectiveness.
- Nature of the Sterilant/Disinfectant:
- Microbicidal capability, speed of action, and residual effects.
- Efficacy in the presence of organic matter such as pus, blood, and feces.
- Duration of Exposure: Longer exposure times generally enhance effectiveness.
- pH: Acidic pH levels can enhance the effectiveness of heat in killing microorganisms.
- Biofilm Formation: The presence of biofilms can hinder the effectiveness of disinfectants.
Classification of Sterilization and Disinfection Methods
A. Physical Methods
Heat:
- Dry Heat: Includes methods like flaming, incineration, and using a hot air oven.
- Moist Heat:
- Below 100°C: Methods such as pasteurization and water baths.
- At 100°C: Techniques like boiling, steaming, and tyndallisation.
- Above 100°C: Sterilization using an autoclave.
Filtration: Involves using depth filters and membrane filters to remove contaminants.
- Radiation:
- Ionizing Radiation: Includes Y-rays, X-rays, and cosmic rays.
- Non-Ionizing Radiation: Involves ultraviolet (UV) and infrared rays.
Ultrasonic Vibration: Uses high-frequency sound waves to disrupt and eliminate contaminants.
B. Chemical Methods
- Alcohols: Ethyl alcohol and isopropyl alcohol are used for their antimicrobial properties.
- Aldehydes: Compounds like formaldehyde, glutaraldehyde, and ortho-phthalaldehyde are effective for disinfection and sterilization.
- Phenolic Compounds: Includes substances like cresol, lysol, chlorhexidine, chloroxylenol, and hexachlorophene, which have antiseptic properties.
- Halogens: Such as chlorine, iodine, and iodophors, are used for their broad-spectrum antimicrobial activity.
- Oxidizing Agents: Hydrogen peroxide and peracetic acid are used for their strong oxidizing and antimicrobial effects.
- Salts: Mercuric chloride and copper salts are used for their antimicrobial properties.
- Surface Active Agents: Quaternary ammonium compounds and soaps are used for their surfactant and antimicrobial properties.
- Dyes: Aniline dyes and acridine dyes are used for their antimicrobial properties.
- Gas Sterilization:
- Formaldehyde is used for gas sterilization at low temperatures.
- Ethylene oxide (ETO) and beta-propiolactone (BPL) are also used for gas sterilization.
Physical Methods of Sterilization/Disinfection
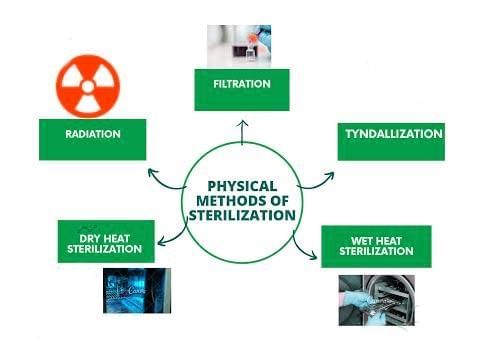
Heat Sterilization/Disinfection
Mechanism of Action
- Dry heat kills organisms by charring, oxidative damage, and denaturing bacterial proteins.
- Moist heat destroys microorganisms by denaturing and coagulating proteins.
Dry Heat (Hot Air Oven)
- Required temperature: 160°C for 2 hours
- Materials that can be effectively sterilised using a hot air oven include:
- Glassware: glass syringes, petri dishes, flasks, pipettes, and test tubes.
- Surgical instruments: scalpels, scissors, forceps, etc.
- Chemicals: liquid paraffin, fats, glycerol, and glove dust powder, etc.
- Sterilisation control:
- Spores (106) of non-toxigenic strains of Clostridium tetani or Bacillus subtilis subsp. niger.
- Thermocouples and Browne’s tube.
Moist Heat at a Temperature below 100ºC
- Pasteurization: Used for perishable drinks like fruit and vegetable juices, beer, and dairy products such as milk.
- Two methods:
- Holder method: 63ºC for 30 minutes.
- Flash method: 72ºC for 20 seconds, then cooled to 13ºC.
- All nonsporing pathogens are killed except Coxiella burnetii, which can survive in the holder method and needs higher temperatures to be eliminated.
- Water bath: This method disinfects serum, body fluids, and vaccines at 60°C for one hour.
- Inspissation (Fractional sterilization):
- This is a process involving heating an item on three consecutive days at 80–85ºC for 30 minutes.
- It is used for sterilising egg-based media and serum-based media (such as Loeffler’s serum slope).
Moist Heat at 100ºC
- Boiling: When items are boiled in water for 15 minutes, most of the vegetative forms are effectively killed, but the spores remain unaffected.
- Steaming: Koch’s or Arnold’s steam sterilizers operate at a temperature of 100°C for 90 minutes. This method is appropriate for media that decompose at higher autoclave temperatures. While it eliminates most vegetative forms, it does not eradicate spores.
- Tyndallization or intermittent sterilization: This technique involves steaming at 100°C for 20 minutes over three consecutive days. It is suitable for sterilising media containing gelatin, egg, serum, or sugar. Tyndallization effectively kills most vegetative forms, but spores are not affected.
Moist Heat at a Temperature above 100ºC (Autoclave)
- Mechanism of action of heat sterilization:
- Dry heat eliminates organisms by increasing levels of electrolytes.
Pasteurization. This method is used for drinks and dairy products like milk:
- Holder method: 63°C for 30 minutes.
- Flash method: 72°C for 20 seconds, then cooled to 13°C.
Section 1
Sterilization Conditions:
- Hot air oven: 160°C for 2 hours.
- Autoclave: 121°C for 15 minutes at a pressure of 15 psi.
Uses of autoclave:
- The autoclave is particularly effective for sterilising:
- Surgical instruments
- Culture media
- Items that cannot withstand the higher temperatures of a hot air oven
- Media containing water that cannot be sterilised by dry heat
Biological indicator: Spores of Geobacillus stearothermophilus (best indicator).
Thermocouple and indicators: Browne’s tube and autoclave tapes.
Filtration: Filtration is effective for removing microbes from heat-sensitive materials such as:
- Vaccines
- Antibiotics
- Toxins
- Serums
- Sugar solutions
Filtration: Filtration is effective for removing microbes from heat-sensitive materials such as:
- Vaccines
- Antibiotics
- Toxins
- Serums
- Sugar solutions
Depth filters: These porous filters hold particles throughout the filter’s depth, rather than just on the surface. They are used in industries for filtering food, drinks, and chemicals but are not suitable for bacterial filtration. Examples include:
- Candle filters made of diatomaceous earth (Berkefeld filters).
- Unglazed porcelain (Chamberland filters).
- Asbestos filters (Seitz and Sterimat filters).
- Sintered glass filters.
Membrane filters: These are commonly used for bacterial filtration. They are porous and trap particles smaller than their pore size on the surface. Made of:
- Cellulose acetate.
- Cellulose nitrate.
- Polycarbonate.
- Polyvinylidene fluoride.
Pore size: Membrane filters typically have an average pore diameter of 0.22 µm.
Filtration of air: Air filters are membrane filters used to provide bacteria-free air. Examples include:
- Surgical masks that allow air in but keep microorganisms out.
In biological safety cabinets and laminar airflow systems:
- HEPA filters: Remove 99.97% of particles ≥ 0.3 µm.
- ULPA filters: Remove 99.999% of particles ≥ 0.12 µm.
Sterilization control: Includes Brevundimonas diminuta and Serratia marcescens.
Ionizing radiation:
- Examples include X-rays, gamma rays (from Cobalt 60 source), and cosmic rays.
- Mechanism: Causes DNA breakage without raising the temperature (known as cold sterilisation).
- It destroys spores and vegetative cells but is not effective against viruses. Used for:
- Disposable plastics like rubber or plastic syringes, infusion sets, and catheters.
- Catgut sutures, bone and tissue grafts, adhesive dressings, antibiotics, and hormones.
Advantages of ionizing radiation:
- High penetrating power.
- Fast action.
- No increase in temperature.
Sterilization control: The effectiveness of ionizing radiation is tested using Bacillus pumilus.
Nonionizing radiation:
- Examples include infrared and ultraviolet radiation.
- They are lethal but do not penetrate glass, dirt, or water.
- Dose: 250–300 nm wavelength for 30 minutes.
- Used for sterilising clean surfaces in operating theatres, laminar flow hoods, and for water treatment.
Chemical Agents of Sterilization/Disinfection
Alcohols
- Alcohols are effective against bacteria, fungi, and some enveloped viruses like HIV, but they do not kill spores.
- They work by altering proteins and possibly disrupting membrane fats.
- Ethyl alcohol, commonly used at 70% concentration, is applied in hand rubs as an antiseptic.
- Isopropyl alcohol is utilized for sterilizing clinical thermometers.
Aldehydes
- Aldehydes act by interacting with nucleic acids and proteins, inactivating them through crosslinking and alkylation.
- They are capable of killing spores and can be used as chemical sterilants.
- Formaldehydeis used for:
- Preserving anatomical specimens
- Fumigating areas such as operation theatres
- Preparing toxoids from toxins, although it is toxic, irritating, and corrosive to metals.
- Glutaraldehyde is less toxic and irritating, making it suitable for sterilizing endoscopes and cystoscopes.
- It is used at a 2% concentration (2% cidex) for 20 minutes and needs to be activated by alkalinisation before use. It remains active for only 14 days.
- Ortho-Phthalaldehyde(0.55%) can also sterilize endoscopes and cystoscopes, offering advantages over glutaraldehyde such as:
- No need for activation
- Lower vapour production
- Better odour
- Greater stability during storage
- Enhanced mycobactericidal activity
Phenolic Compounds
- Phenolic compounds like Cresol, xylenol, Lysol, and ortho-phenylphenol are utilized as disinfectants due to their effectiveness in the presence of organic matter.
- These compounds are toxic and irritating to the skin, making them suitable for disinfection but not for antiseptic use.
- Some phenolics are less irritating and are used as antiseptics, primarily effective against gram-positive bacteria.
- Chlorhexidine, found in products like Savlon (chlorhexidine and cetrimide), is an active ingredient used for its antiseptic properties.
- Chloroxylenol, present in Dettol, is another phenolic compound used for its disinfectant properties.
- Hexachlorophane has limited use due to its potential to cause brain damage and is only recommended for staphylococcal outbreaks.
Halogens
- Iodine is employed as a skin antiseptic, where it acts by oxidizing cell components and iodinating cell proteins. Examples include Tincture of iodine (2%) and Iodophor, such as Betadine, where iodine is complexed with an organic carrier.
- Chlorine is used for disinfection in municipal water supplies, swimming pools, and laboratories. It functions as a bleaching agent to remove stains from clothing. Chlorine is available in various forms, including chlorine gas, sodium hypochlorite (household bleach, 5.25%), and calcium hypochlorite (bleaching powder). All forms produce hypochlorous acid (HClO), which is effective against vegetative bacteria and fungi but not spores.
- Disadvantages of chlorine include:
- Interference by organic matter, necessitating the use of excess chlorine
- Carcinogenic properties
- Requirement for daily preparation
- Ineffectiveness against Giardia and Cryptosporidium
- Corrosiveness of sodium hypochlorite, requiring careful handling
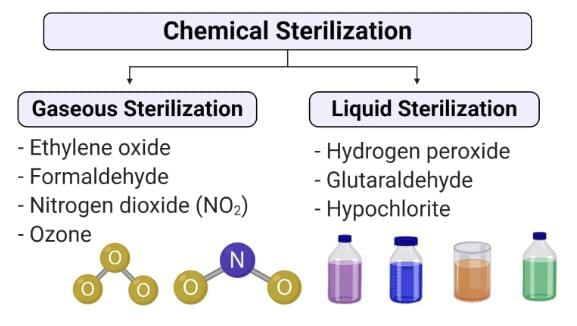
Hydrogen Peroxide (H2O2)
- Plasma Sterilization: Plasma sterilization involves the use of active agents such as hydrogen peroxide (H2O2) and/or peracetic acid. This method is particularly suitable for heat-labile surgical instruments as it maintains a low temperature during the sterilization process. The sterilization process is monitored using Geobacillus stearothermophilus as a control indicator.
- Mode of Action: Hydrogen peroxide acts as a chemical sterilant by releasing toxic free hydroxyl radicals. These radicals are harmful to cell membranes, lipids, DNA, and other cellular components. The ideal concentration of H2O2 for sterilization is typically between 3–6%. However, for catalase-producing organisms and spores, a higher concentration of 10% H2O2 is recommended.
Uses: H2O2 is commonly used to disinfect various medical equipment and devices, including:
- Ventilators
- Soft contact lenses
- Tonometer biprism
Vaporized H2O2 is utilized in plasma sterilization processes.
Advantages of Hydrogen Peroxide:
- Effective in the presence of organic matter.
- Low toxicity levels.
- Environmentally safe.
Peracetic Acid
Peracetic acid is a potent chemical sterilant often used in conjunction with hydrogen peroxide (H2O2) for disinfecting hemodialyzers and in plasma sterilization. It is also effective for sterilizing endoscopes. However, it is important to note that peracetic acid can corrode materials such as steel, iron, copper, brass, and bronze.
Plasma Sterilization
Plasma sterilization is a contemporary method (e.g., Sterrad and Plazlyte) that generates a plasma state while ensuring a uniform vacuum within the chamber.
- Chemical sterilants used in this process include hydrogen peroxide (H2O2) alone or a mixture of H2O2 and peracetic acid.
- The active agents responsible for killing microorganisms and spores are Ultraviolet (UV) photons and radicals such as oxygen (O) and hydroxyl (OH) radicals.
- Low temperatures, typically below 50°C, are maintained during the process, making it suitable for heat-sensitive surgical instruments.
- Sterilization control is achieved using indicators such as Geobacillus stearothermophilus and Bacillus subtilis subsp. niger.
Heavy Metal Salts
- Heavy metal salts have specific applications in certain areas:
- Silver sulfadiazine is utilized for treating burn wounds.
- A 1% silver nitrate solution is employed to prevent ophthalmia neonatorum in newborn infants.
- Copper sulfate serves as an effective fungicide in lakes and swimming pools.
- Mercury salts, such as mercuric chloride, thiomersal, and mercurochrome, were once commonly used antiseptics. Thiomersal (merthiolate) is still used as a preservative in vaccines and sera.
- Mechanism of action: Heavy metals exert their antimicrobial effect by binding to bacterial cell proteins, particularly to their sulfhydryl groups, leading to inactivation. They can also precipitate cell proteins. Many heavy metals are more bacteriostatic than bactericidal in nature.
Surface Active Agents
Surface active agents, or surfactants, lower the surface tension between liquids or between a liquid and a solid. They can act as detergents, wetting agents, and emulsifiers due to their dual nature, possessing both polar hydrophilic and nonpolar hydrophobic ends.
- Cationic surfactants, also known as quaternary ammonium compounds, disrupt microbial membranes and can denature proteins.
- They are effective against most bacteria, particularly gram-positive bacteria, but are not effective against M. tuberculosis or spores.
- Cationic surfactants are non-toxic, but their efficacy can be compromised by acidic pH, organic matter, hard water, and soap.
- Examples of cationic surfactants include:
- Acetyl trimethyl ammonium bromide (cetavlon or cetrimide)
- Alkyltrimethylammonium salts
- Benzalkonium chloride and cetylpyridinium chloride
- Anionic surfactants, such as soaps, have strong detergent properties but weaker antimicrobial effects. They are most effective at acidic pH levels.
- Amphoteric surfactants possess both detergent and antimicrobial properties and are effective across a wide pH range. However, their activity may decrease in the presence of organic matter. For instance, ‘Tego compounds’ are used as antiseptics in dental practice but may cause allergic reactions.
Dyes
Dyes, including aniline and acridine dyes, are commonly used as skin and wound antiseptics.
- Aniline dyes, such as crystal violet, gentian violet, brilliant green, and malachite green, are more effective against gram-positive bacteria than gram-negative bacteria and have no effect against M. tuberculosis.
- These dyes are non-toxic and non-irritating to tissues, although their effectiveness diminishes in the presence of organic material like pus. They disrupt the synthesis of the peptidoglycan component of the bacterial cell wall.
- Aniline dyes are also used in laboratories as selective agents in culture media, such as malachite green in LJ medium.
- Acridine dyes, including acriflavine, euflavine, proflavine, and aminacrine, are minimally affected by organic material and are more effective against gram-positive bacteria, although they are less selective than aniline dyes. They interfere with the synthesis of nucleic acids and proteins in bacterial cells.
Gaseous Sterilization
Ethylene Oxide (ETO)
- Ethylene oxide is a widely used chemical sterilant in gaseous form.
- It effectively penetrates materials and eliminates both microbes and spores by interacting with cell proteins.
- Due to its flammability, irritability, explosiveness, and potential carcinogenicity, it is typically used in concentrations of 10 to 20% mixed with inert gases.
Alkyltrimethylammonium Salts
- Benzalkonium chloride
- Cetylpyridinium chloride
Tego Compounds
Prions are the most resilient structures and require specific methods for their destruction, including:
- Autoclaving at 134°C for 3 to 4 minutes
- Treatment with 1 N NaOH for 1 hour
- Using 0.5% sodium hypochlorite for 2 hours
Sterilization Conditions:
- 5 to 8 hours at 38°C
- 3 to 4 hours at 54°C
Sterilization Control is conducted using Bacillus globigii.
Uses of Ethylene Oxide Sterilization:
- Disposable Plastic Petri Dishes
- Syringes
- Heart-Lung Machines
- Sutures
- Catheters
- Respirators
- Dental Equipment
Microorganism Resistance to Disinfectants
- Prions are the most resistant to disinfectants, followed by:
- Cryptosporidium oocysts
- Coccidian cysts
- Bacterial spores
- Mycobacteria
- Other parasite cysts, such as Giardia
- Small non-enveloped viruses
- Protozoan trophozoites
- Gram-negative bacteria
- Fungi
- Large non-enveloped viruses
- Gram-positive bacteria
- Enveloped viruses (least resistant)
Testing Methods for Disinfectants:
- Phenol coefficient (Rideal Walker) test
- Chick Martin test
- Capacity (Kelsey-Sykes) test
- In-use (Kelsey and Maurer) test
Sporicidal Agents:
- EFGH: Ethylene oxide, Formaldehyde, Glutaraldehyde, Hydrogen peroxide
- 3P: Peracetic acid, O-Phthalic acid, Plasma sterilization
- Autoclave and hot air oven
Testing of Disinfectants

Phenol Coefficient (Rideal-Walker) Test:
- This test compares the effectiveness of a disinfectant to that of phenol in killing Salmonella Typhi.
- A phenol coefficient greater than 1 indicates acceptable effectiveness.
Drawbacks of Phenol Coefficient Test:
- Limited to evaluating phenolic compounds.
- Does not account for the presence of organic materials in assessing disinfectant effectiveness.
Modified Rideal-Walker Test:
- This test version evaluates disinfectants in the presence of organic matter, such as dried yeast or feces, for more realistic conditions.
Capacity (Kelsey-Sykes) Test:
- Assesses how well a disinfectant retains its effectiveness after multiple uses in microbiological settings.
In-use (Kelsey and Maurer) Test:
- Evaluates disinfectant effectiveness during actual hospital use.
Biological Sterilization Indicators
- Clostridium tetani non-toxigenic strain
- B. subtilis subsp. niger
- Brevundimonas diminuta
- Serratia
- Geobacillus stearothermophilus
Methods of Sterilization/Disinfection in Clinical Situations
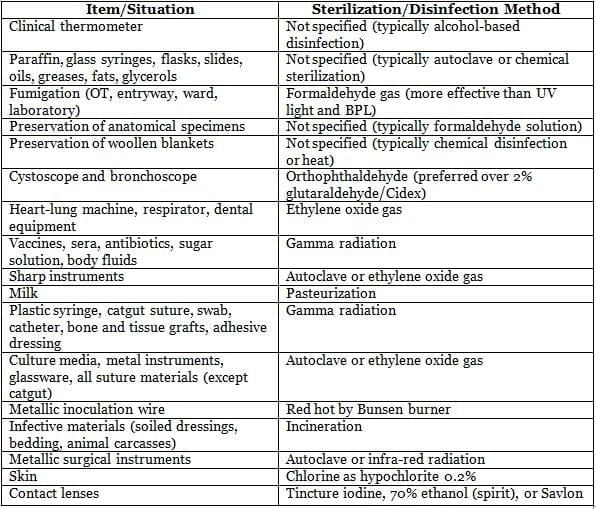
Common Disinfectants and Their Spectrum of Action
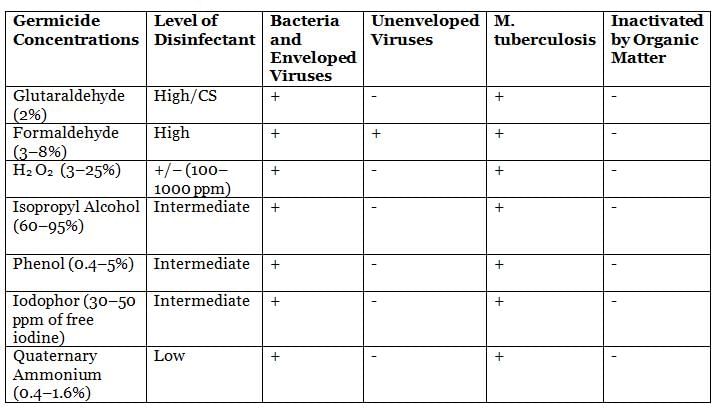
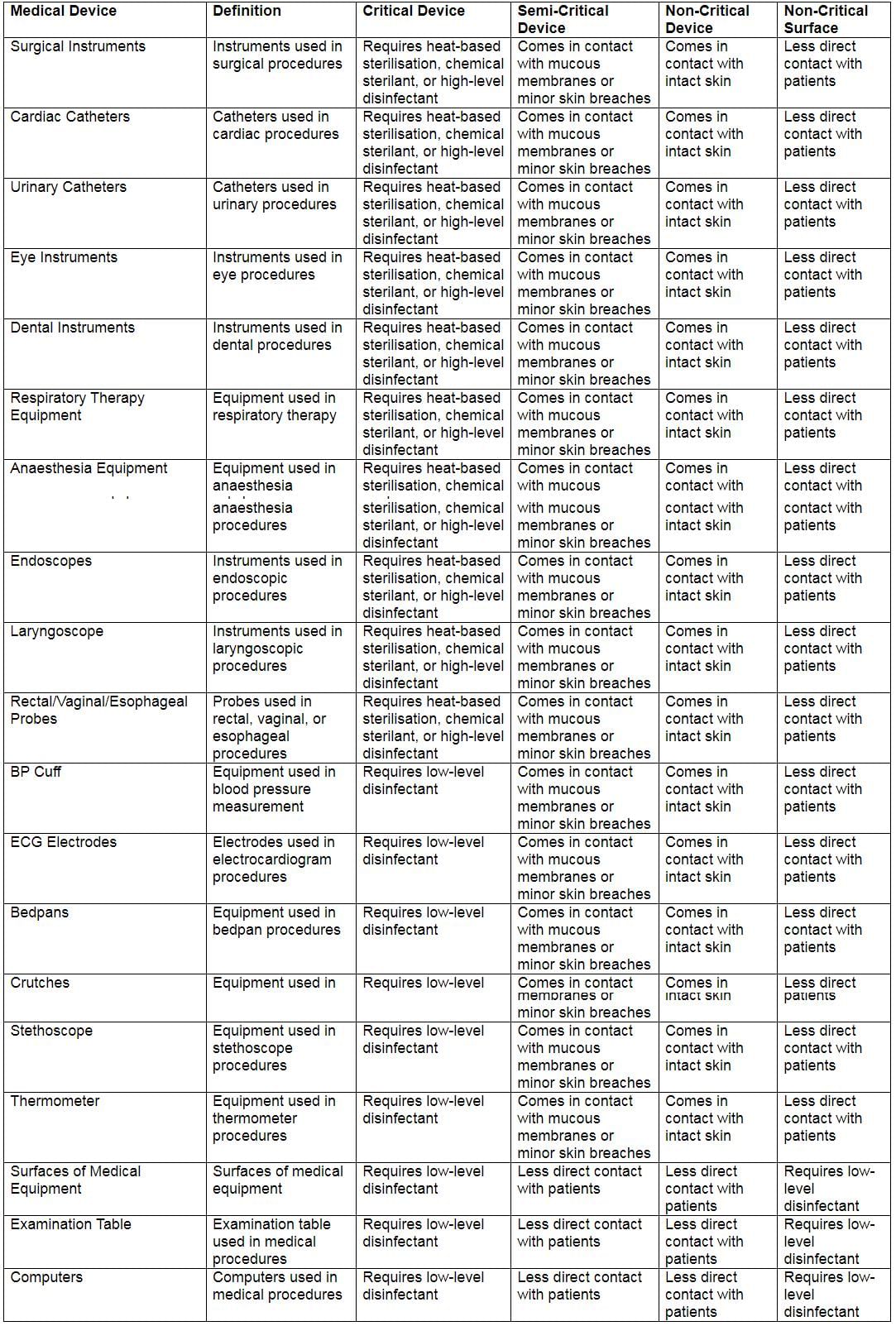
Spaulding’s Classification of Medical Devices
|
75 docs|5 tests
|
FAQs on Sterilization and Disinfection Chapter Notes - Microbiology - NEET PG
| 1. What are the common physical methods of sterilization and disinfection? |  |
| 2. How do chemical agents work in the process of sterilization and disinfection? |  |
| 3. What are the key factors to consider when testing disinfectants? |  |
| 4. What is the difference between sterilization and disinfection? |  |
| 5. Why is it important to properly sterilize and disinfect medical equipment? |  |




















