Structure of Immune System and Immune Response Chapter Notes | Microbiology - NEET PG PDF Download
Structure of Immune System
- Lymphoid Organs
- Central or Primary lymphoid organs: Examples are the thymus and the bone marrow.
- Peripheral or Secondary lymphoid organs: Examples include lymph nodes, spleen, and MALT.
- Lymphoid Cells
- Include lymphocytes such as T-cells, B-cells, and NK cells.
- Other cells of the immune system
- Include phagocytes like macrophages, neutrophils, eosinophils, and basophils.
- Dendritic cells, mast cells, and platelets are also included.
- Cytokines
- These are soluble products secreted by various cells of the immune system.
Central Lymphoid Organs
Bone marrow
Bone marrow is the site where progenitor T- and B-cells start their development. B-cells continue their development in the bone marrow, while progenitor T-cells migrate to the thymus for further maturation.
Thymus
The thymus originates during embryonic development from the third and fourth pharyngeal pouches and is most active at birth. It reaches its maximum size during puberty and gradually shrinks thereafter.
- Structure of the Thymus: The thymus consists of two lobes, each divided into an outer cortex and an inner medulla.
- Cortex: The cortex is densely packed with thymocytes (thymic lymphocytes), epithelial cells, and nurse cells, which are specialized epithelial cells that surround and support thymocytes.
- Medulla: The medulla contains fewer thymocytes and is composed of epithelial cells, dendritic cells, and Hassall’s corpuscles, which are layers of degenerating epithelial cells.
- Thymic Hormones: The thymus produces several thymic hormones, including thymulin, thymopoietin, and thymosin, which play crucial roles in the development and maturation of T-cells.
Maturation of T-cells in the Thymus
- Double Negative (DN) Stage: T-cell precursors entering the thymus become double negative T-cells (CD4– CD8–).
- Double Positive (DP) Stage: DN cells gain CD4 and CD8 markers, becoming double positive T-cells.
- Death by Neglect: The majority (about 95%) of T-cells fail to recognize their specific MHC molecules and undergo apoptosis.
- Positive Selection: The remaining 5% of DP T-cells undergo positive selection, where they are tested for their ability to recognize self-MHC molecules.
- Negative Selection: Among those positively selected, 2–5% are found to be self-reacting and are eliminated to prevent autoimmunity.
- Mature T-cells: The surviving DP T-cells (about 2–5%) lose either CD4 or CD8 markers to become mature T-cells, such as CD4+ helper T-cells or CD8+ cytotoxic T-cells. These mature T-cells acquire thymus-specific antigens and are released into circulation to migrate to peripheral lymphoid organs, where they play a crucial role in immune responses.
Peripheral Lymphoid Organs
- Lymph nodes are small organs shaped like beans and are made up of three sections:
- Cortex: Contains lymphoid follicles and is primarily a B-cell area.
- Medulla: Another region that is also part of the B-cell area.
- Paracortex: This area is where T-cells are located.
- The cortex has two types of lymphoid follicles:
- Primary lymphoid follicles: These appear before the body encounters an antigen. They are smaller and contain resting B-cells.
- Secondary lymphoid follicles: These develop after the body meets an antigen, containing activated B-cells, such as plasma cells and memory B-cells. They consist of:
- Central area: Known as the germinal center.
- Peripheral zone: Also called the mantle area.
- Spleen: The largest secondary lymphoid organ, divided into two parts:
- White pulp: Composed of two sections:
- Periarteriolar lymphoid sheath: A T-cell area.
- Marginal zone: A B-cell area.
- Red pulp: Contains sinusoids filled with red blood cells (RBCs). This is where older and damaged RBCs are destroyed.
- White pulp: Composed of two sections:
- Defect in spleen: The spleen plays a key role in destroying most microbes. If there are problems with the spleen or if it is removed (splenectomy), there is a higher risk of bacterial infections, especially from capsulated bacteria.
Mucosa Associated Lymphoid Tissue (MALT)
MALT (Mucosa-Associated Lymphoid Tissue) is found in the lining of the intestine, respiratory, and urogenital tracts. The intestine has the most research done on its MALT, which is located in different layers of the intestinal wall:
- Submucosa: This layer contains Peyer’s patches, which are made up of lymphoid follicles.
- Lamina propria: This layer has loose groups of lymphocytes and macrophages.
- Epithelial layer:This layer includes:
- Intraepithelial lymphocytes: A few specialized lymphocytes known as γδ T-cells.
- M cells: These are specialized, flat epithelial cells that lack microvilli. They serve as entry points for various microbes like Salmonella, Shigella, Vibrio, and Poliovirus.
- Secretory IgA: This component lines the M cells and provides local or mucosal immunity.
Lymphocytes
Lymphocytes are a type of white blood cell and come in three main categories:
- T lymphocytes
- B lymphocytes
- NK (natural killer) cells
T and B cells can be further divided into:
- Naive lymphocytes: These are cells that have not yet encountered any antigens.
- Lymphoblasts: These cells develop after encountering antigens.
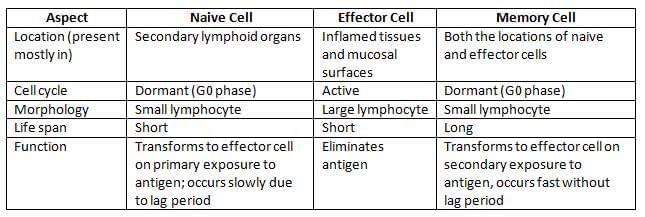
Differences between Naive Cell, Effector Cell, and Memory Cell
T Lymphocytes
There are two main types of effector T-cells:
- CD4+ Helper T-Cells: These cells play a crucial role in assisting other cells of the immune system.
- CD8+ Cytotoxic T-Cells: These cells are responsible for killing infected cells, cancer cells, and sometimes cells that are foreign to the body.
Rare Subtypes of T-Cells: TREG Cells: These regulatory T-cells help the body in several ways:
- They promote tolerance to self-antigens, preventing the immune system from attacking the body’s own tissues.
- They play a role in stopping autoimmune diseases, where the immune system mistakenly attacks healthy cells.
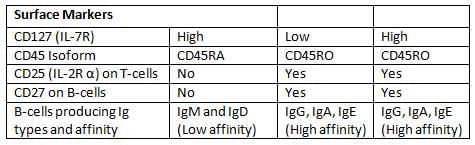
Surface Markers: TREG cells can be identified by specific surface markers, including CD4, CD25, and Foxp3.
IPEX Syndrome: If there is a deficiency of Foxp3 receptors, it can lead to a severe autoimmune condition known as Immune Dysregulation, Polyendocrinopathy, Enteropathy X-linked (IPEX) syndrome.
γδ T-Cells:
These cells are a unique subset of T-cells that make up about 5% of the total T-cell population. Here are some key features:
- They have γ/δ T-cell receptor (TCR) chains instead of the more common α/β chains found in other T-cells.
- Unlike other T-cells, they do not express CD4 or CD8 molecules on their surface.
- They have a different mechanism of action, as they do not require antigen processing or the presentation of peptides by Major Histocompatibility Complex (MHC) molecules.
γδ T-cells are considered part of the innate immune system because their γδ receptors have a limited diversity, meaning they recognize a narrower range of antigens.
- These cells are typically found in the gut mucosa, where they exist as intraepithelial lymphocytes (IELs). Their exact function is still being researched, but they may be involved in interacting with lipid antigens that pass through the intestinal mucosa.
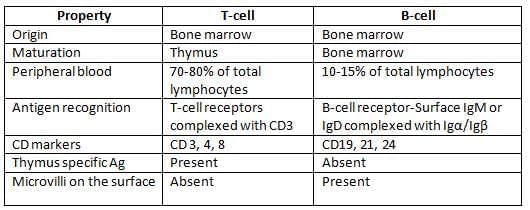
Differences between T-Cell and B-Cell
B Lymphocytes
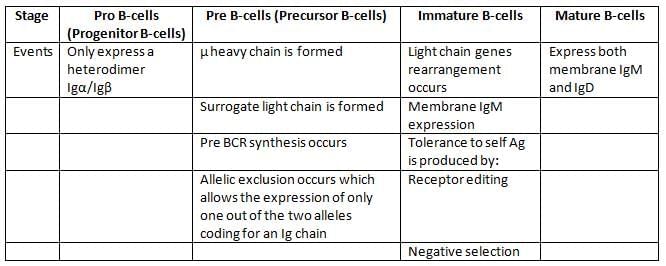
B-cells proliferate through various stages, first in bone marrow, then in peripheral lymphoid organs.
B-cell development in bone marrow is described below.
Development in peripheral lymphoid organs:
- Immature B-cells move from the bone marrow to peripheral lymphoid organs, such as the lymph nodes and spleen.
- In these organs, they change into mature or naive B-cells.
- When they encounter an antigen, these mature B-cells become activated B-cells, also known as lymphoblasts.
- Activated B-cells can further develop into two main types:
- Effector B-cells, which primarily become plasma cells.
- Memory B-cells, which help the immune system remember past infections.
Natural Killer (NK) Cells:
- NK cells are a type of large granular lymphocyte.
- They make up about 10–15% of the total lymphocytes in the body.
- More details about NK cells will be discussed later under Cell-Mediated Immunity (CMI).
Other Cells of the Immune System
- Macrophages
- Dendritic Cells
- Granulocytes (such as neutrophils, eosinophils, and basophils)
- Mast Cells
Macrophages
- Origin: Monocytes and macrophages come from the bone marrow, specifically from a type of cell known as granulocyte-monocyte progenitor cells.
- Size: Monocytes are the largest type of blood cells. They do not multiply and usually move into tissues within 8 hours of entering the bloodstream.
- Differences Between Macrophages and Monocytes
- Macrophages are 5 to 10 times larger than monocytes.
- They have more lysozymes, organelles, enzymes, and cytokines.
- Macrophages are better at phagocytosis (the process of engulfing and digesting pathogens) and can live in tissues for a longer time, from months to years.
Types of macrophages
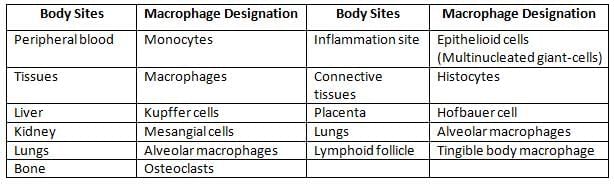 Distribution and function of dendritic cells
Distribution and function of dendritic cells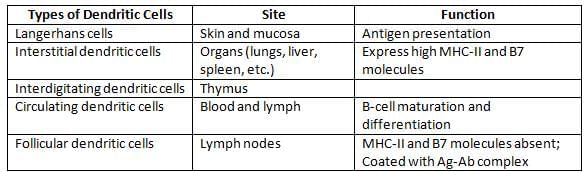
Note: Langerhans cells are a type of dendritic cell found in the skin, while Langhans giant cells are a type of macrophage involved in chronic inflammation.
Major Histocompatibility Complex
- MHC molecules, also known as human leukocyte antigens (HLA), act as unique identification markers for each person because the genetic makeup of MHC genes varies from individual to individual.
- These molecules play a crucial role in determining the histocompatibility between a donor's graft and a recipient.
- In humans, the HLA complex that encodes for MHC proteins is found on the short arm of chromosome 6.
- The genes are organized into three main areas called MHC region I, MHC region II, and MHC region III.
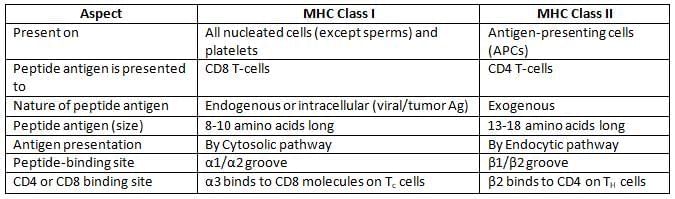
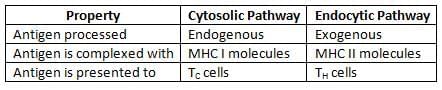
- MHC I and MHC II are essential for presenting antigens to T-cells:
- MHC I displays intracellular antigens from viral or tumor cells to cytotoxic T-cells.
- MHC II shows extracellular antigens on antigen-presenting cells (APCs) to helper T-cells.
- MHC III does not aid in antigen presentation but instead codes for various proteins, including:
- Complement factors such as C2, C4, C3 convertase, factor B, and properdin.
- Heat shock proteins.
- Factors like TNF-α and TNF-β.
- Steroid 21-hydroxylases.
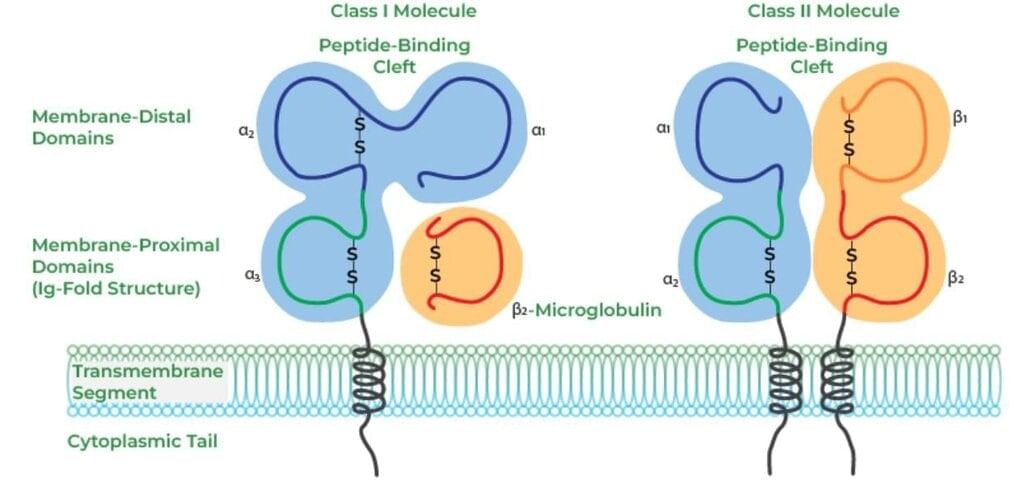
Differences between MHC Class I and MHC Class II Molecules
Cytokines: Sources and Functions
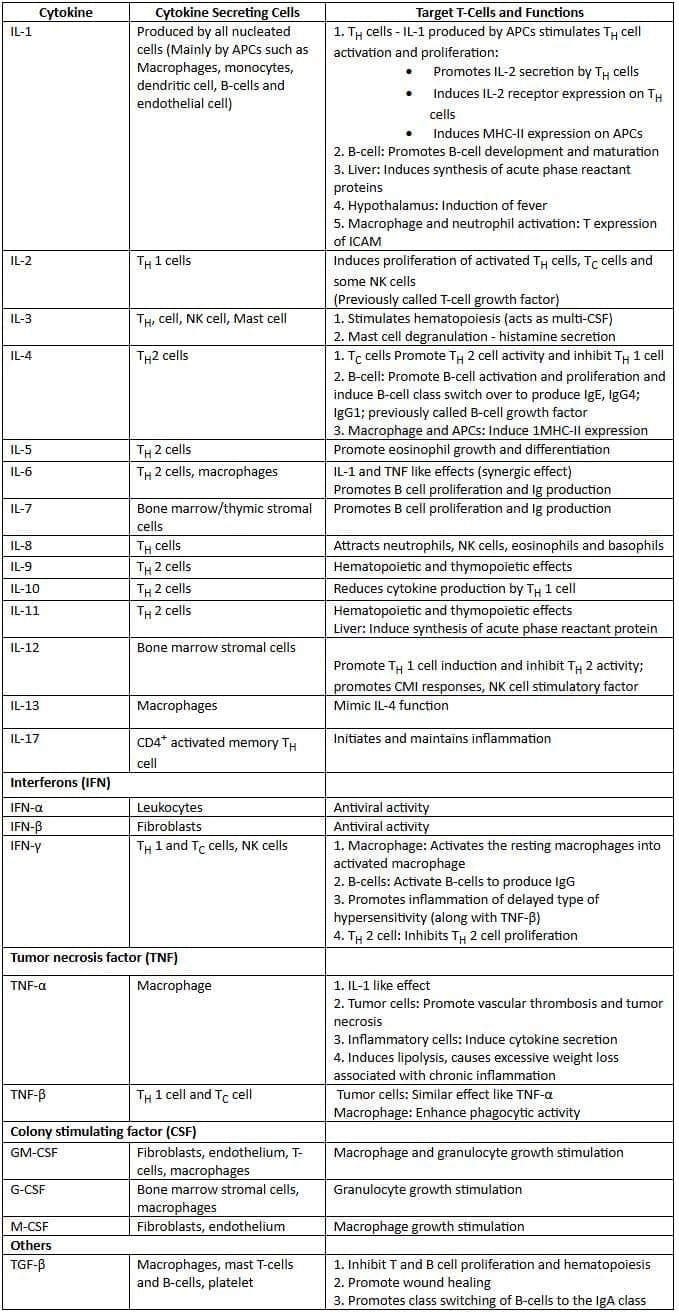
Immune Responses
The immune response is a coordinated reaction involving cells of the immune system and their products. It has two parts: Humoral or Antibody Mediated Immune response (AMI) and Cell-Mediated Immune response (CMI).
Interaction of CMI and AMI
- CMI and AMI function independently but are interconnected, regulated by helper T (TH) cells.
- Both CMI and AMI share a common pathway where antigens are presented to helper T-cells, leading to their activation.
Activation of Helper T-Cells- Activated helper T-cells differentiate into TH1 or TH2 subsets.
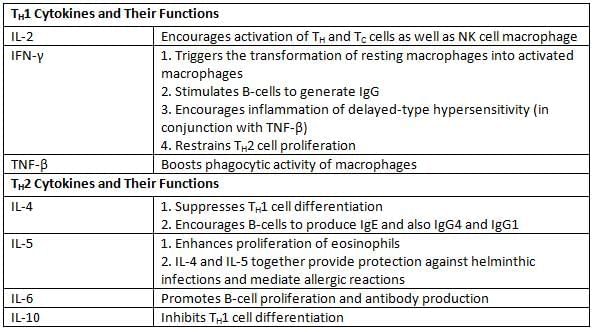
- TH1 cells release cytokines that promote CMI.
- TH2 cells secrete cytokines that encourage B-cells to produce antibodies.
Antigen-Presenting Cells (APCs)
- APCs are specialized cells that play a crucial role in the immune response by presenting antigenic peptides to T helper (TH) cells.
- They do this by displaying these peptides on their surface using MHC class II molecules.
- APCs can be categorized into two main types:
- Professional APCs: These are highly efficient in antigen presentation and include cells such as macrophages, dendritic cells, and B-cells.
- Non-professional antigen-presenting cells: These cells can also present antigens, but they are not as specialized as professional APCs. Examples include fibroblasts (found in the skin), thymic epithelial cells, pancreatic beta cells, vascular endothelial cells, glial cells (in the brain), and thyroid epithelial cells.
Antigen Processing Pathways
Helper T-cells (TH Cells) Activation and Differentiation
Activation of TH Cells
- Antigen-specific signal: Binding of an antigenic peptide on antigen-presenting cells (APCs) to the T-cell receptor (TCR) of TH cells.
- CD4 molecules: Interaction with the β2 domain of MHC-II.
- Costimulatory signal: Binding of CD28 on TH cells to B7 molecules on APCs.
- Cytokine signal: IL-1 influencing TH cell responses.
TH1 Cytokines and Their Functions
Cell-Mediated Immune Response
Cell-Mediated Immune Response
- Cell-Mediated Immunity (CMI) protects against:
- Microbes that live inside cells (both types: obligate and facultative)
- Cancer cells
- Delays or triggers type IV hypersensitivity
- Has an important role in transplant immunity and reactions between grafts and hosts
- Effector cells involved in CMI include:
- Antigen-specific cells:
- Cytotoxic T-cells (main cells responsible for CMI)
- Antigen-nonspecific cells:
- Natural Killer (NK) cells
- Cells that perform ADCC (including NK cells, macrophages, neutrophils, and eosinophils)
- Antigen-specific cells:
Cytotoxic T Lymphocytes
CD8 T-cells are the main effector cells for CMI. To become active, Naive T-cells need specific signals:- Antigen-specific signal: The T-cell receptor (TCR) on naive T-cells attaches to the MHC I-peptide complex on target cells.
- CD8 interaction: The CD8 molecule on T-cells also connects with a part of MHC-I.
- Costimulatory signal: The CD28 molecule on naive T-cells interacts with the B7 molecule found on target cells.
- Cytokine signal: Interleukin-2 (IL-2), released by TH1 cells, affects T-cells.
- Once activated, T-cells produce two kinds of destructive enzymes:
- Perforins: Create pores in target cells.
- Granzymes: Lead to the destruction of target cells.
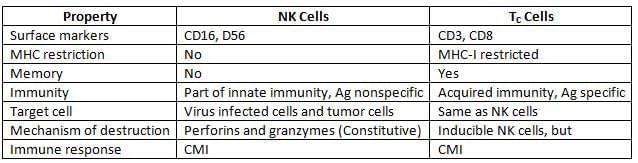
Natural Killer (NK) Cells:
NK cells are large granular lymphocytes constituting 10–15% of peripheral blood lymphocytes. They originate from a different lymphoid lineage and exhibit cytotoxic activity without antigen specificity.
- Role in Innate Immunity: NK cells are part of innate immunity, acting as a first line of defense. They do not require prior contact with an antigen to exert their cytotoxic effects.
- Mechanism of NK Cell-Mediated Cytotoxicity:
- Receptor Interaction: Activation receptors on NK cells, such as NKR-P1 and CD16, engage with ligands on target cells, leading to NK cell activation.
- Target Cell Destruction: NK cells secrete perforins and granzymes to induce apoptosis in target cells. Unlike TC cells, NK cell enzymes are constitutively present, allowing them to be cytotoxic without prior antigen exposure.
Comparison between NK Cells and TC Cells:
 Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
Antibody-Dependent Cell-Mediated Cytotoxicity (ADCC)
- A variety of nonspecific cytotoxic cells have receptors called FcR on their surfaces that can attach to the Fc region of any antibody.
- These cells can connect to the Fc portion of antibodies that are attached to target cells, leading to the destruction of those target cells through the release of different cytotoxic factors, such as:
- NK cells (Natural Killer cells) release perforins and granzymes.
- Neutrophils secrete lytic enzymes.
- Eosinophils can release both lytic enzymes and perforins, which help protect against helminths.
- Macrophages produce lytic enzymes and TNF (Tumor Necrosis Factor).
- Even though these cytotoxic cells do not specifically target an antigen, the specificity of the antibody guides them to the right target cells. This kind of cytotoxicity is known as antibody-dependent cell-mediated cytotoxicity (ADCC).
- The assessment or detection of Cell-Mediated Immunity (CMI) can be done through:
- Mixed-lymphocyte reaction (MLR)
- Cell-mediated lympholysis (CML)
- The graft-versus-host reaction (GVH) in experimental animals.
Humoral/Antibody Mediated Immune Response (AMI)
AMI protects the host by producing antibodies that stop microbes from invading the host's cell surfaces and the spaces outside the cells. However, it does not help against microbes that are inside the cells.
AMI happens in three main steps:
- Step 1: Activation of B-cells that carry microbial antigens. These B-cells act as Antigen-Presenting Cells (APCs) and show the antigens to T Helper (TH) cells. This step needs:
- Signal 1: This is created when the microbial antigen links with the membrane-bound immunoglobulin (mIg) on the B-cell.
- Signal 2: This comes from the CD40 on the B-cell binding with CD40L (ligand) found on activated TH cells.
- Signal 3: The cytokines made by the activated TH cells act on the B-cells.
- Step 2: Differentiation of B-cells into effector cells (plasma cells) and memory cells. This process takes place in the germinal center of secondary lymphoid follicles.
Cytokines responsible for Ig class/subclass switch over
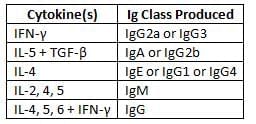
- Effector functions: Plasma cells produce secreted antibodies that play a crucial role in fighting off microbes.
- These antibodies can work in several ways, including:
- Neutralization: Antibodies can bind to pathogens, preventing them from entering or damaging cells.
- Opsonization: Antibodies mark microbes for destruction by other immune cells, making it easier for them to be eliminated.
- Complement activation: Antibodies can trigger a series of proteins that help destroy pathogens.
- ADCC (Antibody-Dependent Cellular Cytotoxicity): Antibodies attract immune cells to kill infected cells.
- Mucosal immunity: Antibodies help protect mucosal surfaces, such as those in the gut and respiratory tract, from infections.
|
75 docs|5 tests
|
FAQs on Structure of Immune System and Immune Response Chapter Notes - Microbiology - NEET PG
| 1. What are the primary components of the immune system? |  |
| 2. How does the innate immune response differ from the adaptive immune response? |  |
| 3. What role do T cells and B cells play in the immune response? |  |
| 4. What are antibodies, and how do they function in the immune system? |  |
| 5. What is the significance of memory cells in the immune response? |  |
















