Study Of The First Element – Hydrogen Chapter Notes | Chemistry Class 9 ICSE PDF Download
Introduction
Imagine a tiny element that powers stars, fuels rockets, and is a key part of the water we drink every day. That’s hydrogen, the first element in the periodic table and a fascinating subject of study! This chapter takes you on an exciting journey through hydrogen’s unique position in the periodic table, its preparation methods, and its role in chemical reactions. From fizzing experiments in the lab to its industrial production, hydrogen’s story is one of versatility and importance. Let’s dive into the world of this lightweight, reactive element and uncover its secrets!
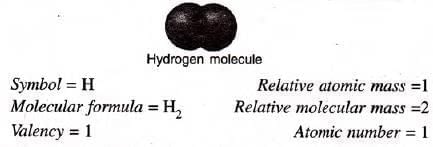
Electronic configuration : 1 (K shell) Position in Periodic Table : 1st Period; 1A Group
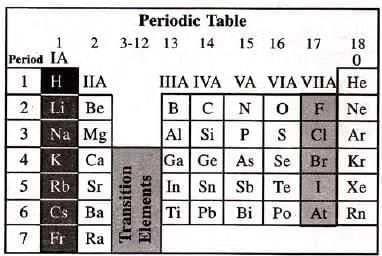
Position of Hydrogen in Periodic Table
- Hydrogen is the first element in the periodic table with atomic number 1.
- It has one electron in its valence shell, placing it in the 1st period and Group IA.
- Its properties don’t fully match Group IA (alkali metals) or Group VIIA (halogens).
- It shows a dual nature, resembling both alkali metals and halogens.
- Due to its unique properties, some suggest placing it separately at the top of the periodic table.
Similarities Between Hydrogen and Alkali Metals
- Electronic Configuration: Hydrogen and alkali metals have one electron in their outermost shell.
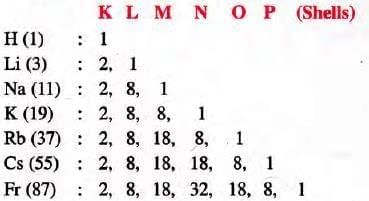
- Valence Electrons: All have one valence electron in their outermost shell.
- Valency: Hydrogen and alkali metals have a valency of 1.
- Ion Formation: They form positive ions by losing one electron.
- H → H+ + e-
- Li → Li+ + e-
- Na → Na+ + e-
- Reducing Power: Both act as reducing agents.
- CuO + H2 → Cu + H2O
- CuO + 2Na → Cu + Na2O
- Burning: Hydrogen burns in oxygen to form water.
- 2H2 + O2 → 2H2O (burns with a pop sound)
- Alkali metals burn in oxygen to form oxides:
- Lithium: 4Li + O2 → 2Li2O (monoxide)
- Sodium:2Na + O2 → Na2O2 (peroxide)
- Potassium: K + O2 → KO2 (superoxide)
- Action of Air: Alkali metals tarnish in air due to oxide, hydroxide, and carbonate formation.
- 4Na + O2 → 2Na2O
- Na2O + H2O → 2NaOH
- 2NaOH + CO2 → Na2CO3 + H2O
- Combination with Non-Metals: Both react with non-metals like oxygen, sulphur, and chlorine.
- Hydrogen: Forms H2O, H2S, HCl
- Sodium: Forms Na2O, Na2S, NaCl
Similarities Between Hydrogen and Halogens
- Electronic Configuration: Hydrogen and halogens have one electron less than the nearest inert gas.
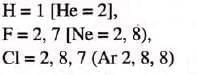
- Valency:
- Both have valency 1.
- Thus, they accept one electron to attain the electronic configuration o f the nearest inert gas.
Formation of ions: Both show a tendency to form anions since they are one electron short o f the nearest inert gas configuration.
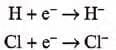
- Electronegative character: Both halogens and hydrogen are non-metals. They show electronegative character.
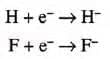
- Physical State: Hydrogen and halogens (F, Cl) are gases.
- Atomicity: Exist as diatomic molecules (H2, F2, Cl2, Br2, I2).
Properties of Hydrogen Different From Alkali Metals and Halogens
- Hydrogen has only one electron shell, while alkali metals and halogens have two or more.
- Hydrogen’s oxide (H2O) is neutral, unlike acidic halogen oxides (Cl2O, Cl2O7) or basic alkali metal oxides (Na2O, K2O).
Discovery
- Henry Cavendish discovered hydrogen in 1766, though Robert Boyle prepared it in 1672.
- Cavendish prepared it from iron and dilute acids, calling it “inflammable air.”
- Lavoisier named it “hydrogen” in 1783, meaning “water former” due to its water-forming ability.
Occurrence
Free State:
- Found in traces in earth’s crust (0.98%), atmosphere (0.01%), volcanic gases (0.025%).
- Sun and stars contain 1.1% hydrogen.
Combined State:
- Present in plant and animal tissues (with carbon, oxygen, nitrogen).
- Found in acids, alkalis, hydrocarbons, proteins, sugars, starch, petroleum, fats.
- Constitutes 11.1% of water by weight.
Preparation of Hydrogen
Hydrogen is prepared from water, acids, or alkalis due to their hydrogen content.
Hydrogen from Water
From Cold Water and Metals:
Reactive metals (K, Na, Ca) react with cold water, forming hydroxides and hydrogen (exothermic).
- Potassium:
- Floats on water (density 0.86 g/cc), melts at 62°C, forms a silver-grey globule.
- Reaction: 2K + 2H2O → 2KOH + H2 (highly exothermic).
- Burns with a lilac flame due to potassium vapour; produces soapy, alkaline solution.
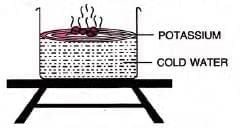
- Caution: Stored in kerosene, handled with tongs due to reactivity.
- Sodium:
- Floats (density 0.97 g/cc), melts at 97°C, forms silvery globule.
- 2Na + 2H2O → 2NaOH + H2 (less exothermic than K).
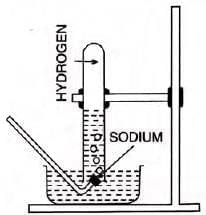
- Burns with a golden yellow flame; forms soapy, warm, alkaline solution.
- Note: Sodium amalgam reacts smoothly with water for safe hydrogen preparation.
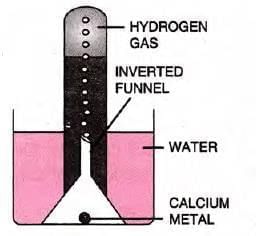
- Calcium:
- Sinks in water; less vigorous reaction.
- Reaction: Ca + 2H2O → Ca(OH)2 + H2
- Forms milky, turbid, alkaline solution (turns red litmus blue).
From Hot Water and Steam:
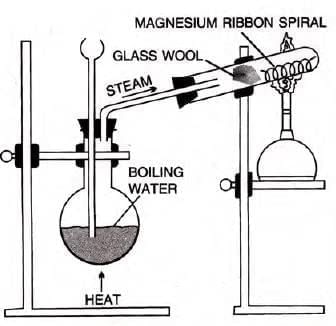
- Magnesium:
- Magnesium reacts slowly with boiling water and forms a base, magnesium hydroxide, liberating hydrogen gas.
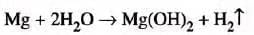
- Magnesium burns in steam with an intense white light, liberating hydrogen gas and white ash, i.e., magnesium oxide.
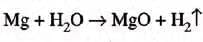 Magnesium oxide cru m b les dow n due to heating, further exposing magnesium to steam results in the liberation of hydrogen gas.
Magnesium oxide cru m b les dow n due to heating, further exposing magnesium to steam results in the liberation of hydrogen gas.
- Aluminium:
- Reacts with steam at 800°C: 2Al + 3H2O → Al2O3 + 3H2
- Forms oxide coating, halting reaction unless coating breaks at high temperature.
- Zinc:
- Reacts with steam when heated: Zn + H2O → ZnO + H2
- Forms zinc oxide (yellow when hot, white when cold).
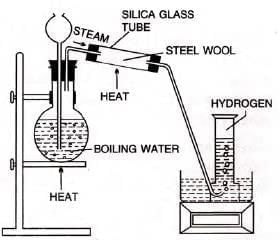
- Iron:
- Red-hot iron reacts reversibly with steam: 3Fe + 4H2O ⇌ Fe3O4 + 4H2
- Equilibrium at 700°C; forward reaction slows as products form.
Note:
- In the beginning, the forward reaction is fast and the backward reaction is slow, since the speed of a reaction is directly related to the masses (or the concentrations) of the reacting substances.
- As the reactants get consumed, the forward reaction becomes slower.
- In other words, as the products are formed, the backward reaction becomes faster.
- There comes a time when the forward and backward reactions acquire the same speed.
- Thereafter, the reaction does not appear to move in a single direction, and the amounts of the reactants and the products do not change.
- This equilibrium stage is attained at 700°C.
When metals react with steam:
(i) Rate of reaction is fastest in the case of magnesium but it progressively decreases in the cases of aluminium, zinc, and iron.
(ii) In the case of magnesium, aluminium, and zinc, the reactions stop after some time. This is because the oxides of these metals stick to the surfaces of the respective metals and thus do not allow steam to come into contact with the metal.
- From Steam on Non-Metals:
- Carbon (coke) with steam forms water gas: C + H2O → CO + H2
Application of Activity Series in the Preparation of Hydrogen
- Activity series ranks metals by reactivity:
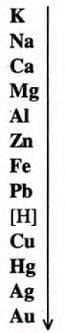
- Potassium is most reactive; gold is least.
- Hydrogen, a non-metal, is included due to its ability to form H+.
- Reactivity decreases down the series; metals above hydrogen displace it from water/acids.
Salient features of the Activity Series
- Electropositive character decreases down the series.
- Reducing power decreases; potassium is the strongest reducing agent.
- Tendency to lose electrons (oxidation) decreases.
- Metals above hydrogen displace it from water/acids:
- 2Na + 2H2O → 2NaOH + H2
- Zn + 2HCl → ZnCl2 + H2
- Metals below hydrogen (Cu, Ag) don’t displace hydrogen:
- Cu + H2O → No reaction
- Cu + HCl → No reaction
- Higher metals displace lower ones from salt solutions:
- CuSO4 + Zn → ZnSO4 + Cu
- Oxides of K, Na, Ca, Mg, Al require electrolysis for reduction.
- Oxides below Al reduce with H2, CO, or C:
- ZnO + C → Zn + CO
- PbO + H2 → Pb + H2O
- Oxides/nitrates of Hg, Ag, Au decompose on heating:
- 2HgO → 2Hg + O2
- 2AgNO3 → 2Ag + 2NO2 + O2
- Metals below copper resist rusting.
- Top metals form chlorides (Na, K), middle form oxides (Al), lower form sulphides (Zn, Pb, Cu).
Displacement of Hydrogen from Dilute Acids
Metals above hydrogen displace H2 from dilute HCl or H2SO4.
Reactivity order:
- K, Na, Ca: Explosive reaction.
- Mg, Al, Zn: Vigorous but safe reaction.
- Fe, Ni, Sn, Pb: Gentle reaction.
- Cu, Hg, Ag, Au: No reaction.
Reactions:
- Mg + 2HCl → MgCl2 + H2
- 2Al + 3H2SO4 → Al2(SO4)3 + 3H2
- Zn + H2SO4 → ZnSO4 + H2
- Fe + 2HCl → FeCl2 + H2
Why Zinc is Preferred:
- Na, K react violently.
- Ca, Mg are costly.
- Al forms protective Al2O3 coating.
- Fe requires heating, produces impure H2 (H2S, SO2).
- Pb forms insoluble PbSO4/PbCl2, halting reaction.
- Metals below H (Cu, Hg) don’t displace H2.
Displacement of Hydrogen by Alkalis
Zinc, lead, aluminium (amphoteric) react with hot concentrated alkalis to produce H2.
Reactions:
- Zinc:
- Zn + 2NaOH → Na2ZnO2 + H2 (Sodium zincate)
- Zn + 2KOH → K2ZnO2 + H2 (Potassium zincate)
- Lead:
- Pb + 2NaOH → Na2PbO2 + H2 (Sodium plumbite)
- Pb + 2KOH → K2PbO2 + H2
- Aluminium:
- 2Al + 6NaOH → 2Na3AlO3 + 3H2 (Sodium aluminate)
- 2Al + 2NaOH + 2H2O → 2NaAlO2 + 3H2 (Sodium meta aluminate)
- 2Al + 2KOH + 2H2O → 2KAlO2 + 3H2 (Potassium meta aluminate)
- Amphoteric Nature: Zn, Pb, Al oxides/hydroxides react with both acids and bases.
- ZnO + 2HCl → ZnCl2 + H2O
- ZnO + 2NaOH → Na2ZnO2 + H2O
Laboratory Preparation of Hydrogen
Reactants: Granulated zinc, dilute HCl or H2SO4.
Procedure:
- Use a flat-bottom flask with an airtight cork having two holes.
- Insert a thistle funnel and delivery tube through the holes.
- Add dilute HCl/H2SO4 through the funnel.
Reactions:
- Zn + 2HCl → ZnCl2 + H2
- Zn + H2SO4 → ZnSO4 + H2
Why Granulated Zinc: Copper impurity in granulated zinc catalyzes the reaction.
Observation: Effervescence and gas evolution occur.
Collection: By downward displacement of water because:
- Hydrogen is nearly insoluble in water (20 mL dissolves in 1 L).
- Forms explosive mixture with air, so not collected by air displacement.
Impurities present with hydrogen:
- hydrogen sulphide (H2S)
- sulphur dioxide (SO2)
- oxides of nitrogen
- phosphine (PH3)
- arsine (AsH3)
- carbon dioxide
- water vapour
Impurity Removal:
Silver nitrate solution: Removes AsH3, PH3.
- AsH3 + 6AgNO3 → Ag3As + 3AgNO3 + 3HNO3
- PH3 + 6AgNO3 → Ag3P + 3AgNO3 + 3HNO3
Lead nitrate solution: Removes H2S.
- Pb(NO3)2 + H2S → PbS + 2HNO3
Caustic potash (KOH) solution: Removes SO2, CO2, NOx.
- SO2 + 2KOH → K2SO3 + H2O
- CO2 + 2KOH → K2CO3 + H2O
- 2NO2 + 2KOH → KNO2 + KNO3 + H2O
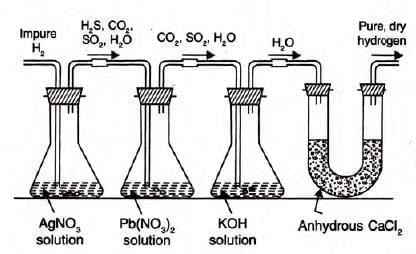
Drying agents (fused CaCl2, KOH stick, P2O5): Remove water vapour.
Final Collection: Over mercury, as it doesn’t react with hydrogen.
Concentrated sulphuric acid is a good drying agent but it is not used to dry hydrogen as it reacts with hydrogen, thus de eating the purpose of the reaction.
H2SO4 + H2 → 2H2O + SO2
Precautions:
- Ensure airtight apparatus to prevent leaks.
- No flames nearby due to H2-air explosion risk.
- Collect gas only after air is expelled (test by burning gas quietly).
- Thistle funnel end must dip in acid to prevent gas escape.
Note:
Nitric acid, even in its dilute form, is not used in preparation of hydrogen from metals because:
Nitric acid is a powerful oxidizing agent, and the oxygen formed due to its decomposition oxidizes the hydrogen to give water, thus defeating the purpose of the reaction.
- Oxidizes H2 to H2O: 3Zn + 8HNO3 → 3Zn(NO3)2 + 4H2O + 2NO↑
Magnesium [Mg] and manganese [Mn] are the only metals that react with very dilute nitric acid (1% nitric acid) to liberate hydrogen. The oxidizing action of the acid is much reduced due to its overdilution.
- Mg, Mn react with 1% HNO3:Mg + 2HNO3 → Mg(NO3)2 + H2↑
Concentrated sulphuric acid is not used in preparation of hydrogen as it will produce sulphur dioxide.
- Zn + 2H2SO4 → ZnSO4 + SO2 + 2H2O
Test for Hydrogen:
- Combustible but doesn’t support combustion.
- Burns silently with pale blue flame: 2H2 + O2 → 2H2O
- Explodes with pop sound when premixed with air/oxygen.
Manufacture of Hydrogen
Bosch Process:
- Step 1: Steam over hot coke (1000°C) forms water gas (endothermic).
- C + H2O → CO + H2
- Step 2: Water gas + steam passed over Fe2O3 (catalyst) and Cr2O3(promoter) (exothermic).
- (CO + H2) + H2O → CO2 + 2H2
- Step 3: CO2removal:
- Passed through cold water (30 atm) or KOH solution.
- 2KOH + CO2 → K2CO3 + H2O
- Step 4: CO removal:
- Passed through ammoniacal CuCl solution.
- CuCl + CO + 2H2O → CuCl.CO.2H2O
By Electrolysis:
- Acidulated water (H2O + H2SO4) electrolysed.
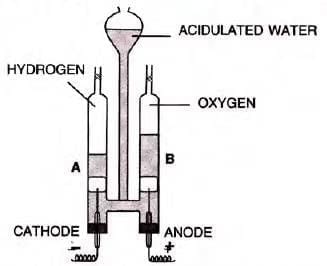
- H2 at cathode: H+ + e- → H; H + H → H2
- O2 at anode: OH- - e- → OH; OH + OH → H2O + O; O + O → O2
- Overall: 2H2O → 2H2 + O2
- Advantage: Pure H2 and O2 produced.
Properties of Hydrogen
Physical Properties

Occlusion: Adsorbed by Pd, Pt, Ni (e.g., 1 vol Pd adsorbs 900 vol H2).
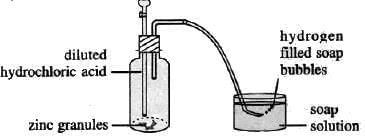
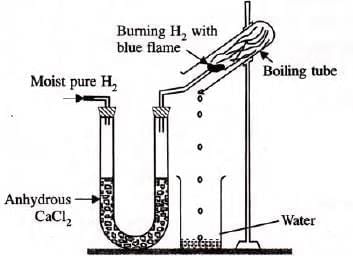
To prove that hydrogen is a very light gas
Experiment: Take soap solution in a beaker and allow hydrogen to pass through it.
On passing hydrogen gas through the soap solution, soap bubbles filled with hydrogen fly higher and burst out. This behaviour proves that hydrogen is lighter than air.
Chemical Properties
- Reactive with non-metals, metals, oxides, organic compounds.
- Doesn’t support combustion.
With Non-Metals:
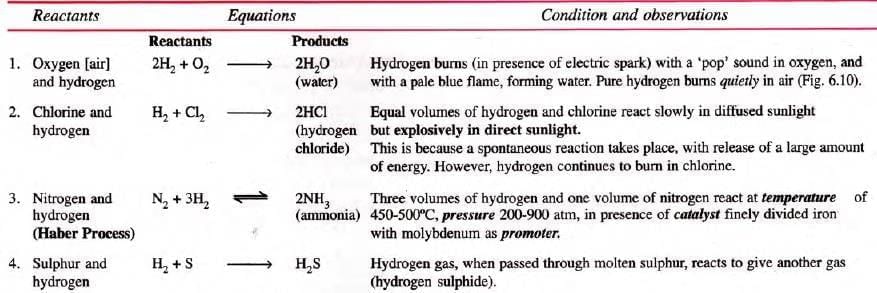
Note: Hydrogen forms an explosive mixture with air (due to the oxygen present in air). If the amount of air is less in the mixture, the explosion is not dangerous, i.e. the gas bums with a pop sound only. But a large volume of mixture causes a dangerous explosion leading to serious damage and injuries. (Hydrogen-oxygen mixture is called detonating mixture).
Reaction of hydrogen with metals:

Reaction with metallic oxides:
- Hydrogen gas is a strong reducing agent.
- Hydrogen reduces the oxides of the less active metals, i.e. it removes oxygen from strongly heated metal oxides when passed over them and itself gets oxidized to water.
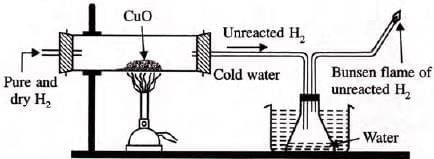
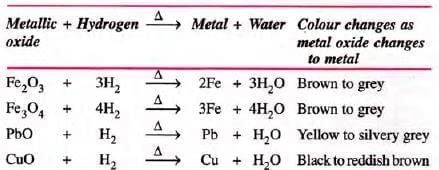
- Oxides of Na, K, Al, Ca, Mg not reduced due to high oxygen affinity.
- Note: H2-O2 mixture is explosive (detonating mixture).
Uses of Hydrogen
- As a fuel: Because of its high heat of combustion, hydrogen is used as a fuel in the form of:
(i) Coal gas,
(ii) Water gas (CO + H₂),
(iii) Liquid hydrogen (non-polluting and easy to store). - Oxy-hydrogen torch: A mixture of hydrogen and oxygen is burnt in a specially-designed apparatus called an oxy-hydrogen torch to produce temperatures touching 2500°C. The flame is used for cutting and welding metals, for melting platinum and quartz, and for fusing alumina to produce synthetic rubies and sapphires that are used as jewels in watches.
Atomic hydrogen torch: Creates high temperature (2800°C), which is used for welding alloys containing metals like tungsten, manganese and chromium.
In self-lighting gas jets and automatic lighters.
In manufacture of ammonia (Haber Process), hydrogen chloride, methyl alcohol:
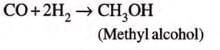
For hydrogen of vegetable oil: Hydrogen is used in preparation of solid vanaspati ghee from liquid vegetable fats like groundnut oil, coconut oil, etc. This process is called catalytic hydrogenation of oils because it takes place in the presence of finely-divided nickel or platinum or palladium as the catalyst. This process occurs at high pressure and at a temperature of about 200°C.
For producing artificial petrol from coal: Passage of hydrogen under high pressure over coal in presence of a catalyst is known as hydrogenation of coal. It produces a product similar to petrol but containing a higher proportion of hydrogen.
In extraction of metals: Hydrogen reduces the metallic oxide (less active metal oxide).
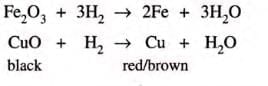
Oxidation and Reduction
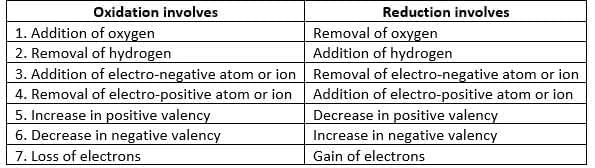
Oxidation
- Addition of oxygen: 2Mg + O2 → 2MgO
- Removal of hydrogen: H2S + Cl2 → 2HCl + S
- Addition of electronegative ion:2FeCl2 + Cl2 → 2FeCl3
- Removal of electropositive ion: 2KI + Cl2 → 2KCl + I2
- Increase in positive valency.
- Decrease in negative valency.
- Loss of electrons: Zn → Zn2+ + 2e-
Reduction:
- Removal of oxygen:CuO + H2 → Cu + H2O
- Addition of hydrogen: Cl2 + H2S → 2HCl + S
- Removal of electronegative ion:2FeCl3 + H2S → 2FeCl2 + 2HCl + S
- Addition of electropositive ion: 2HgCl2 + SnCl2 → Hg2Cl2 + SnCl4
- Decrease in positive valency.
- Increase in negative valency.
- Gain of electrons: Cu2+ + 2e- → Cu
Reducing Agents:
Provide electrons, hydrogen, or remove oxygen.
Examples:
- Solids: C, Zn, Al, Cu, Na, SnCl2, glucose.
- Liquids: H2O2, HI, HBr.
- Gases: H2S, CO.
Tests:
- Brown NO2 fumes with HNO3.
- CuO (black) to Cu (red) on heating.
- Decolourises KMnO4 (pink).
- K2Cr2O7 from orange to green.
- Fe(III) (yellow) to Fe(II) (green).
Redox Reactions:
Oxidation and reduction occur simultaneously.
- Example:CuSO4 + Zn → ZnSO4 + Cu
- Oxidation: Zn → Zn2+ + 2e-
- Reduction: Cu2+ + 2e- → Cu
- Another example: CuO + H2 → Cu + H2O
- H2 (reducing agent) reduces CuO to Cu.
- CuO (oxidizing agent) oxidizes H2 to H2O.
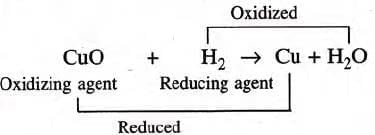
- In this reaction, hydrogen, the reducing agent, reduces the oxidizing agent Cu (II) oxide to copper. On the other hand, oxidation reaction is the oxidation of the reducing agent, hydrogen, to water, by the oxidizing agent, Cu (II) oxide. Thus the net reaction is a redox reaction.
- In the reactiorr tretwfeeir nynrogefi' su lp h id e 'tmn chlorine, hydrogen sulphide loses its hydrogen to chlorine and is thereby oxidized to sulphur; and chlorine, which gains hydrogen, is reduced to hydrochloric acid.
Differences between oxidation and reduction
Points to Remember
- Hydrogen is in 1st period, Group IA, with dual nature (resembles IA and VIIA).
- Found in traces in earth’s crust, atmosphere; abundant in stars (1.1%).
- Present in water, acids, alkalis, hydrocarbons, proteins, tissues.
- Prepared from water using K, Na, Ca (cold water), Mg (hot water), Zn, Fe (steam).
- Displaced from dilute HCl/H2SO4 by metals above H in activity series.
- Zn, Pb, Al produce H2 with acids and alkalis (amphoteric).
- Lab preparation: Zn + dilute HCl/H2SO4, collected by water displacement.
- Impurities (H2S, SO2, etc.) removed using AgNO3, Pb(NO3)2, KOH, drying agents.
- Mg, Mn produce H2 with 1% HNO3.
- Industrial methods: Electrolysis, Bosch process (steam + coke).
- Lightest gas, burns with pop sound, adsorbed by Pd.
- Reacts with metals, non-metals; reduces metal oxides (CuO, PbO).
- Hydrogen is used as a fuel as well..
|
10 videos|47 docs|9 tests
|
FAQs on Study Of The First Element – Hydrogen Chapter Notes - Chemistry Class 9 ICSE
| 1. What is the position of hydrogen in the periodic table and why is it unique? |  |
| 2. What are the similarities between hydrogen and alkali metals? |  |
| 3. How does hydrogen resemble halogens, and what are the implications of these similarities? |  |
| 4. What are the common methods for the laboratory preparation of hydrogen? |  |
| 5. What are the main applications of hydrogen in various industries? |  |