Water Chapter Notes | Chemistry for SSS 2 PDF Download
Introduction
Water is very important for all living things on Earth. It covers 71% of the Earth's surface and is needed for survival. Our body has about 70% water, which helps in keeping us cool, digesting food, and moving nutrients to different parts of the body. We use water every day for things like drinking, bathing, cleaning, and growing plants. It is also used in industries and to produce electricity. This chapter will teach us about water, its properties, how it dissolves things, its sources, how to clean it, and why it is so special.
Water – A Revision
- Water covers 71% of the Earth’s surface and is very important for all living things to survive.
- Most life processes in living beings happen with the help of water.
- About 70% of the human body is made of water, which helps in cooling the body by removing waste through sweat.
- Water also helps in digesting food and moving nutrients to different parts of the body.
Water is Extensively Used
- We use water at home for bathing, cleaning, washing, and other daily tasks.
- Water is needed for farming and industrial activities.
- It helps in producing electricity by turning turbines, also called hydroelectricity.
Did You Know?
- Fruits and vegetables have a lot of water, which helps us meet our daily water needs.
- Water has a high specific heat, meaning it can take in or lose a lot of heat before its temperature changes. This helps mammals control their body temperature.
Uses of Water
- Plants need a lot of water to make their food through a process called photosynthesis.
- Water helps move minerals to different parts of plants.
- It is used to cool down machines in power plants that run on fossil fuels and in car radiators.
- Water is used for transporting goods and for keeping things clean.
- We need water for fun activities like swimming, river rafting, and boating.
Sources of Water
The main sources of water are rainwater, surface water, and groundwater.
Rainwater
- Rainwater is the purest type of water because it comes from water vapor in the air that turns into liquid.
- When it falls, it can get dirty because of air pollution, so rainwater can also become polluted.
- Rainwater is important because it keeps the level of groundwater steady.
Surface Water
- Surface water is found in oceans, seas, rivers, lakes, streams, and ponds.
- Water in oceans and seas has a lot of dissolved salts, so it is salty and not good for drinking, washing, or farming.
- Water in rivers, lakes, streams, and ponds is freshwater. It comes from melted snow on mountains or from rain.
- As it flows through hills and plains, it picks up dirt, so it needs to be cleaned before use.
Groundwater
- Groundwater is water that collects under the ground after rainwater seeps down through layers of soil and rocks.
- The level of groundwater is called the water table, and it changes depending on the place and season.
- We can get groundwater using wells, tube wells, and handpumps.
- It is clean because it gets filtered while passing through layers of rocks and sand, so it has no dirt.
- Natural springs also have groundwater.
Activity 8.1
Aim: To show that rainwater has no dissolved dirt, but well water has dirt.
Materials Required: Two porcelain dishes, two beakers, some water, a sample of rainwater, a sample of well water, two burners.
Procedure:
- Take samples of rainwater and well water in two porcelain dishes.
- Place the dishes over two beakers with water and heat them until the water evaporates.
Observation: No residue is left in the dish with rainwater, but there are solid rings in the dish with well water.
Conclusion: This shows that rainwater has no dissolved dirt, but well water has dirt that stays behind after the water evaporates.
Water Cycle
- The water cycle is the continuous movement of water between land, water bodies, and the air.
- Water on the Earth’s surface in oceans, seas, rivers, lakes, and other water bodies evaporates because of the sun’s heat and rises into the air.
- Plants also release water into the air through a process called transpiration.
- Respiration by living organisms releases water into the air through breathing:

- Burning of fuels like fossil fuels also releases water:

- High up in the air, water vapor cools and turns into tiny water droplets because the air becomes too cold to hold it.
- These droplets come together to form clouds. When they get too heavy, they fall as rain, hail, or snow.
- The water cycle is a continuous process that keeps water moving between land, air, and living things.
Potable Water
- We know that water we drink must be clean, clear, have no color or smell, and be free from dirt.
- Potable water is water that is safe to drink and has minerals that our body needs.
- Water from natural sources has dirt like clay, sand, dead plants, sewage, germs, and other pollutants, which are called water pollutants.
- Human waste, animal waste, and industrial waste are the main reasons for water pollution.
- To make water safe for drinking, we must remove all the dirt from it at water treatment plants.
Purification of Water
Purification of water is the process of removing harmful chemicals, dirt, and germs from water to make it safe. The main steps to purify water from lakes or rivers are sedimentation, filtration, and chlorination.
Sedimentation
- Water from sources like rivers or lakes is collected in big tanks and left to stand for a few hours.
- Heavy dirt like sand settles at the bottom as sediments.
- We add chemicals like potassium alum and slaked lime (calcium hydroxide) to make the dirt settle faster.
- After sedimentation, the clearer water at the top is sent to filtration tanks without disturbing the dirt at the bottom.
Filtration
- In filtration, water with small dirt particles is passed through a filter to clean it.
- The dirt gets stuck in the filter, and clean water comes out.
- When purifying water on a large scale, it is filtered through layers of sand, gravel, and activated charcoal to remove dirt.
- This step removes dirt that did not settle during sedimentation.
Chlorination
- After filtration, the water might still have harmful germs that can make us sick.
- To kill these germs, the filtered water is treated with chlorine, which is called chlorination.
- Adding chlorine kills bacteria and other germs, making the water safe to drink.
- The process of removing germs from water is also called sterilization.
Purification of Water by Distillation
- Distillation is a process where water is heated to turn it into vapor, and then the vapor is cooled back into liquid to remove dirt.
- Impure water is kept in a distillation flask and heated using a burner.
- The water turns into steam, leaving the dirt behind in the flask.
- The steam passes through a part called Liebig’s condenser, where it cools down and turns back into liquid water.
- The clean water is collected in a receiver, and the dirt stays in the flask.
- Distillation gives us very pure water because it removes both dirt and dissolved solids.
Uses of Distilled Water
- Distilled water is used in car batteries and inverters.
- It is used to make medicines.
- It is used in experiments in science labs.
Purification of Water at Home
We can purify water at home using simple methods to make it safe for drinking.
- Boiling: Boiling is the easiest way to clean water at home. When we boil water, it kills most of the germs in it, making it safe to drink. After boiling, the water should be cooled and filtered to remove any leftover dirt. Boiled water should be kept in a clean, covered container.
- Chlorination: In this method, we add chlorine tablets to water to kill germs and make it safe for drinking.
- Water Purifiers: Water purifiers use ultraviolet rays or a combination of methods to kill germs in water. They make water safe for drinking by removing dirt and germs.
- Addition of Alum: If the water is muddy, we can add a crystal of alum to it. The alum makes the dirt settle at the bottom of the container. Then, we can filter the water and use it.
Physical Properties of Water
A molecule of water is made of two hydrogen atoms and one oxygen atom, so its formula is H₂O. Some properties of water are:
Nature
- Pure water is clear, has no color, no smell, and no taste at room temperature.
- Water has dissolved minerals and gases that give it a pleasant taste.
Boiling Point
- Pure water boils at 100°C under normal air pressure and turns into steam.
- The boiling point changes with pressure:
- When pressure decreases, the boiling point of water goes down. This is why water boils at a lower temperature in hilly areas, where air pressure is less, making it take longer to cook food.
- When pressure increases, the boiling point of water goes up. This is why a pressure cooker cooks food faster because the pressure inside raises the boiling point of water.
Freezing Point
- Pure water freezes at 0°C under normal air pressure and turns into ice.
- The freezing point changes with pressure:
- When pressure increases, the freezing point of water goes down.
- If there are impurities in water, its freezing point goes down, and its boiling point goes up.
Density
- Most liquids expand when heated and shrink when cooled, but water behaves differently.
- Water shrinks when cooled until it reaches 4°C. After that, it expands when cooled below 4°C.
- This unusual behavior is called the anomalous expansion of water.
- Because of this, water is most dense at 4°C, but when cooled below 4°C to 0°C, its density becomes less, and it freezes into ice.
- The density of ice at 0°C is less than the density of water, which is why ice floats on water.
Heat and Electrical Conductivity
- Pure water does not conduct heat or electricity well.
Specific Heat
- Water has a high specific heat, meaning it can take in or release a lot of heat slowly.
- Water does not heat up quickly or cool down quickly compared to other liquids.
- This property makes water a great coolant, so it is used in car engines, factories, and nuclear reactors to cool things down.
- The cooling effect of water helps control the Earth’s climate.
- In coastal areas, breezes blow from the sea to the land during the day (sea breeze) and from the land to the sea at night (land breeze) because water heats up and cools down more slowly than land.
- During the day, the land gets hotter faster than the sea, so cooler air from the sea moves toward the land as a sea breeze.
- At night, the land cools faster than the sea, so warmer air from the land moves toward the sea as a land breeze.
Activity 8.2
Aim: To find the boiling point of water.
Materials Required: Some distilled water, a beaker, a tripod stand, a burner, a thermometer.
Procedure:
- Pour some distilled water into a beaker.
- Set up the apparatus as shown, making sure the bulb of the thermometer dips in the water but does not touch the sides of the beaker.
- Note the temperature of the water when it starts boiling.
Observation: Water starts boiling at 100°C, and the temperature stays constant.
Conclusion: This shows that the boiling point of pure water is 100°C.
Activity 8.3
Aim: To observe the effect of changing pressure on the freezing point of water.
Materials Required: Two ice cubes.
Procedure:
- Take two ice cubes and press them together by applying pressure on both cubes.
- Then, release the pressure and see what happens.
Observation: When you apply pressure, the ice cubes join together to form one big cube. When the pressure is released, the water freezes into ice again, joining the two ice cubes together.
Conclusion: This shows that when pressure is released, the water freezes into ice again. This process is called regelation.
Activity 8.4
Aim: To show that ice is lighter than water.
Materials Required: A glass, a few ice cubes, some water.
Procedure:
- Take a glass and add a few ice cubes to it.
- Now, add water to the glass.
Observation: The ice cubes immediately come to the surface of the water and start floating.
Conclusion: This shows that ice is lighter than water.
Effects of Anomalous Expansion of Water
- The unusual expansion of water helps aquatic animals in cold areas survive.
- Water cools faster at the surface than at deeper levels, so the surface freezes into ice first, which is lighter and floats.
- The deeper water stays at 4°C, which is the heaviest temperature for water, so aquatic animals can live there safely.
- This property helps aquatic life survive in very cold conditions.
Dissolution of Salts in Water
- Water can dissolve many minerals and nutrients that our body needs.
- These dissolved things help the body get rid of waste through urine.
- Water with dissolved salts is filtered by the kidneys in our body.
- When a salt like sodium chloride dissolves in water, it splits into tiny parts called ions.
- The reaction is: NaCl (s) → Na⁺ (aq) + Cl⁻ (aq).
- This splitting is a chemical change because the salt turns into Na⁺ and Cl⁻ ions.
- These ions help water conduct electricity, so we call it a chemical change.
- But when something like glucose dissolves in water, it does not split into ions.
- Glucose stays as molecules in water, so there is no chemical change, only a physical change.
- So, ionic compounds (like salts) cause a chemical change when dissolved, but covalent compounds (like glucose) do not.
Water—A Universal Solvent
- Water is called a universal solvent because it can dissolve many solids, liquids, and gases.
- A substance that dissolves in water is called a solute.
- The mixture of water and the dissolved substance is called a solution.
- Some solids that dissolve in water are salt, sugar, and other ionic and organic compounds.
- Minerals dissolved in water give it a taste, like calcium or magnesium.
- Liquids like alcohol, acids, and bases can also dissolve in water.
- Gases like oxygen, carbon dioxide, ammonia, hydrogen chloride, and sulfur dioxide can dissolve in water.
- Oxygen dissolved in water helps fish breathe, and carbon dioxide in water helps make fizzy drinks.
Solubility
- Solubility means how much solute can dissolve in a solvent at a certain temperature to make a saturated solution.
- There are three types of solutions based on how much solute is dissolved:
- Unsaturated Solution: A solution where more solute can dissolve at that temperature.
- Saturated Solution: A solution where no more solute can dissolve at that temperature.
- Supersaturated Solution: A solution that has more solute than it can normally hold at that temperature.
Activity 8.5
Aim: To show that many substances can be dissolved in water.
Materials Required: Five glasses, water, sodium chloride, sodium carbonate, sodium bicarbonate, copper sulphate, sugar.
Procedure:
- Take five glasses and fill each one halfway with water.
- Put one teaspoon of each substance in separate glasses: sodium chloride in the first glass, sodium carbonate in the second glass, sodium bicarbonate in the third glass, copper sulphate in the fourth glass, and sugar in the fifth glass.
- Stir the mixture in each glass with a spoon.
Observation: All the substances completely dissolve in water, and the solution becomes clear and transparent in each glass.
Conclusion: Water can dissolve many substances.
Factors Affecting the Formation of a Solution
Three things affect how fast a solution forms:
- Size of the Particles: Smaller particles dissolve faster because they have more surface area to mix with water.
- Stirring: Stirring makes the solute mix faster with water, speeding up the dissolving process.
- Increase in Temperature: Heating the solution makes the solute dissolve faster because the particles move quicker.
Activity 8.6
Aim: To show that stirring, heating, and small particle size increase the rate of solution formation.
Materials Required: Five beakers, water at room temperature, hot water, powdered sugar, small lumps of sugar.
Procedure:
- Take five beakers and label them A, B, C, D, and E.
- In beaker A, take water at room temperature and powdered sugar.
- In beaker B, take water at room temperature and small lumps of sugar.
- In beaker C, take hot water with lumps of sugar.
- In beaker D, take hot water with powdered sugar.
- In beaker E, take hot water at room temperature and powdered sugar, but do not stir after adding the sugar.
- Stir the sugar in beakers A, B, C, and D.
Observation: Sugar in beaker A dissolves faster than in beaker B because of smaller particles. Sugar in beaker D dissolves faster than in beakers C and E because of heat and stirring.
Conclusion: Stirring, heating, and smaller particle size make the solution form faster.
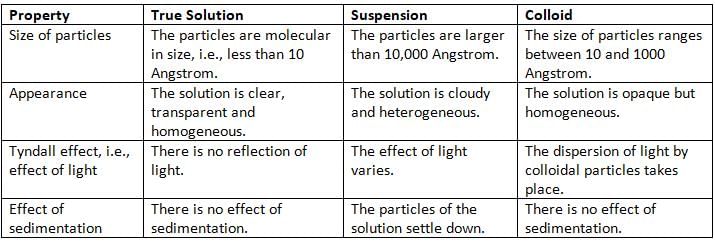
True Solution, Suspension and Colloid
Solutions are classified into three types based on the size of the solute particles:
- True Solution: The solute particles are very tiny, less than 10 Angstrom. It is clear and transparent, like sugar in water.
- Suspension: The solute particles are larger than 10,000 Angstrom. It is cloudy, and particles settle down, like oil in water or mud in water.
- Colloid:The solute particles are between 10 and 1,000 Angstrom in size. It looks clear but is actually cloudy, like smoke, fog, milk, or paint.
- Colloids show the Tyndall effect: When light passes through a colloid, the particles scatter the light, making the beam visible, like in smoke or fog.
Comparison between True Solution, Suspension and Colloid
Water of Crystallisation
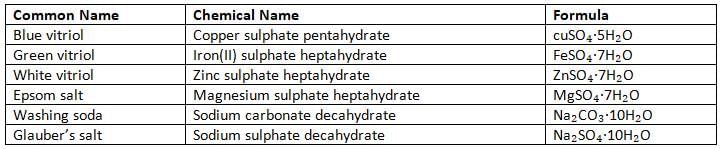
- Some salts have a fixed amount of water in their crystals, called water of crystallisation.
- This water helps the salt form its crystal shape and gives it colour.
- Water of crystallisation is the fixed number of water molecules in the crystal structure of a salt.
- When the salt is heated, it loses this water, and the crystals can be reformed by adding water again.
- For example, iron(II) sulphate (FeSO₄) has 7 water molecules and forms green crystals: FeSO₄·7H₂O.
- When heated, it loses the water and becomes a powder: FeSO₄.
- Salts can have 1, 2, 3, 5, 7, 10, or 12 water molecules in their crystals (called mono, di, tri, penta, hepta, octa, nona, or deca hydrates).
- Salts with water of crystallisation are called hydrated salts.
- When heated, these salts lose their water of crystallisation and become anhydrous salts (without water).
- Some substances absorb moisture from the air but not enough to form a solution; these are called hygroscopic substances.
- Examples of hygroscopic substances: Calcium chloride (CaCl₂), phosphorus pentoxide (P₂O₅), concentrated sulphuric acid (H₂SO₄), concentrated hydrochloric acid (conc. HCl), silica gel.
- Some anhydrous salts like anhydrous copper sulphate and anhydrous sodium carbonate are also hygroscopic.
- Hygroscopic substances are used as drying agents to remove moisture from other substances without reacting with them chemically.
- For example, silica gel packets are used in bottles to absorb moisture and keep things dry.
- Hygroscopy: The process of absorbing moisture from the air without becoming a solution.
- Some substances absorb so much moisture that they form a solution; this is called deliquescence.
- Examples of deliquescent substances: Calcium chloride (CaCl₂·6H₂O), iron(III) chloride (FeCl₃), potassium hydroxide (KOH), magnesium chloride (MgCl₂).
- For example, drying tubes are packed with CaCl₂ to dry gases.
- Silica gel is used in many industries to keep things dry by absorbing moisture.
- It is often found in small packets in new leather items or electronic items to keep them dry.
- If silica gel gets wet, it may not work well and can cause damage by letting moisture in.
- Some substances lose their water of crystallisation when exposed to air; this is called efflorescence.
- For example, FeSO₄·7H₂O loses its water and becomes FeSO₄ when exposed to air.
- Similarly, Na₂SO₄·10H₂O loses 9 water molecules and becomes Na₂SO₄·H₂O when exposed to air.
Some Salts Containing Water of Crystallisation
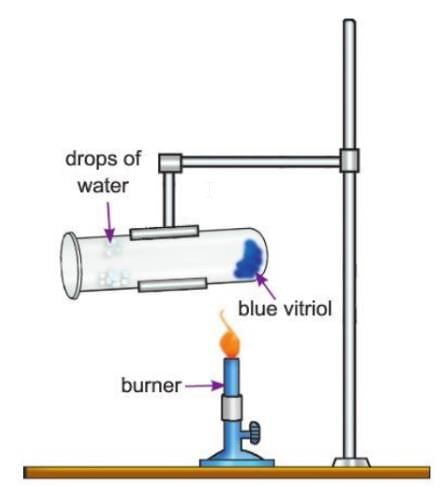
Activity 8.7
Aim: To show that copper sulphate crystals contain water of crystallisation.
Materials Required: Test tube, blue copper sulphate crystals, test tube holder, burner.
Procedure:
- Take a dry test tube and put some blue copper sulphate crystals in it.
- Hold the test tube with a test tube holder so that drops of water do not slip back and crack the tube when it gets hot.
- Heat the test tube carefully over a burner.
Observation: Drops of a colourless liquid form on the upper cooler part of the test tube, and the blue crystals turn into a white powder.
- Cool the test tube and let the colourless liquid drops trickle back onto the white powder.
Observation After Cooling: The white powder turns blue again, showing the colourless liquid is water.
Conclusion: Copper sulphate crystals contain water of crystallisation, and the blue colour comes from this water.
Chemical Properties of Water
Let's study the chemical properties of water:
Nature
- Water is neutral, which means it does not change the color of litmus paper.
Stability
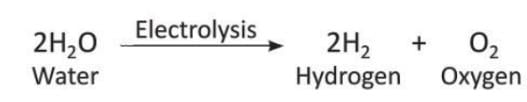
- Water is stable and does not break down easily.
- Even when heated above 2000°C, water does not break into smaller parts.
- But when an electric current is passed through water, it breaks into hydrogen and oxygen gases.
Did You Know?
John Ritter (1768-1810) confirmed that water is made of two parts hydrogen and one part oxygen in 1800. The amount of hydrogen and oxygen in water was measured by Ritter. Water’s electrolysis was discovered in 1781 by Henry Cavendish.
Reactivity with Metals
Reaction of Metals with Cold Water
- Metals react differently with water depending on their position in the reactivity series.
- Metals above magnesium in the reactivity series react strongly with cold water and can catch fire.
- Metals like potassium (K), calcium (Ca), and sodium (Na) react with cold water to form metal hydroxides and hydrogen gas.
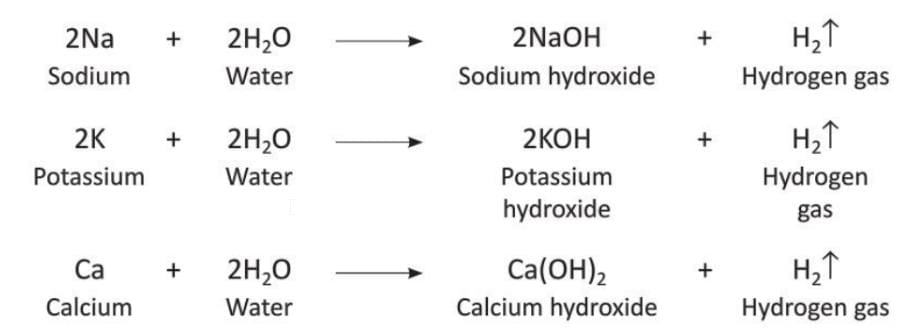
- Metals like magnesium (Mg), aluminum (Al), zinc (Zn), iron (Fe), tin (Sn), lead (Pb), copper (Cu), mercury (Hg), silver (Ag), and gold (Au) do not react with cold water.
Reaction of Metals with Hot Water
Magnesium reacts slowly with hot water to form magnesium hydroxide and hydrogen gas.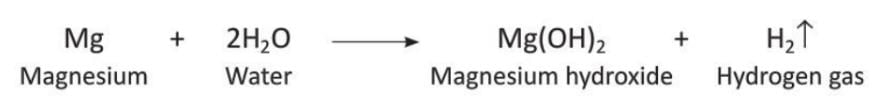
Reaction of Metals with Steam
- Magnesium reacts strongly with steam to form magnesium oxide and hydrogen gas, creating a bright white light.
- Zinc and iron also react with steam but the reaction is less strong.
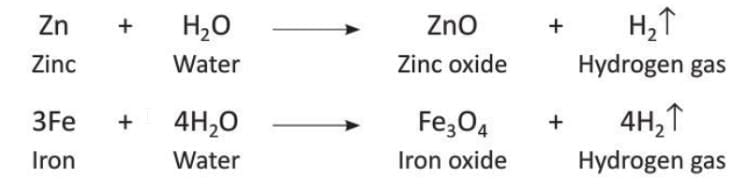
- Aluminum reacts with steam to form aluminum oxide and hydrogen gas, but the reaction stops after a short time because a thin layer of aluminum oxide forms on the metal and stops further reaction.

- Metals like lead (Pb), copper (Cu), mercury (Hg), silver (Ag), and gold (Au) do not react with water in any form.
Hard Water and Soft Water
- Salts in water affect how it works with soap.
- If water makes good lather with soap when shaken, it is called soft water.
- If water does not make lather easily and forms a white layer (scum), it is called hard water.
- Hard water contains salts like calcium or magnesium that stop soap from making lather.
- Soft water does not have these salts, so it makes lather easily with soap.
Activity 8.8
Aim: To test the quality of tap water and distilled water.
Materials required: A pan, a gas, four cups of water.
Procedure:
- Boil 4 cups of tap water in a pan for 20-25 minutes.
- Turn off the flame and observe carefully.
- Now boil 4 cups of distilled water in a pan for 20-25 minutes.
- Turn off the flame and observe carefully.
Observation: In the case of tap water, you will see a white layer formed on the inner surface of the pan. In the case of distilled water, you will see that no white layer is formed.
Conclusion: The white layer in tap water is due to impurities, which means tap water is hard water. No impurities are present in distilled water, so it is soft water.
Activity 8.9
Aim: To observe the formation of scum in hard water and soft water.
Materials required: Two cups of distilled water, some Epsom salt, some liquid detergent, two bottles.
Procedure:
- Pour 1 cup of distilled water in a bottle and add a teaspoon of Epsom salt to it, then add a few drops of liquid detergent. Label it bottle 1.
- Screw the bottle cap tightly and shake the bottle vigorously for 10 seconds.
- In the other bottle, pour 1 cup of distilled water and add a few drops of liquid detergent. Label it bottle 2.
- Screw the bottle cap tightly and shake the bottle well for 10 seconds.
Observation: In bottle 1, adding Epsom salt to distilled water makes it hard water, so you will observe the formation of scum. In bottle 2, you will observe the formation of lather on the surface of the water.
Conclusion: This shows that the water in bottle 2 is soft water because it forms lather.
Causes of Hardness of Water
- Hardness in water is caused by dissolved salts like calcium chloride (CaCl₂), calcium sulphate (CaSO₄), calcium bicarbonate (Ca(HCO₃)₂), magnesium chloride (MgCl₂), magnesium sulphate (MgSO₄), and magnesium bicarbonate (Mg(HCO₃)₂).
- Hardness of water is of two types: temporary and permanent.
- Temporary hardness of water is due to bicarbonates of calcium and magnesium. Temporary hardness can be removed by boiling or filtration, as bicarbonates turn into insoluble carbonates.
- Permanent hardness of water is due to chlorides and sulphates of calcium and magnesium. Permanent hardness cannot be removed by boiling because chlorides and sulphates are stable to heat.
Disadvantages of Hard Water
- Hard water is not good for drinking and cooking purposes.
- Hard water is not good for washing as it wastes soap, forms scum, and leaves dirty stains on clothes.
- Scum deposits as scales on metals, making them lose their shine in hard water.
- Hard water is not good for use in factories and industries as it forms scales that damage machines and waste fuel.
Removal of Hardness of Water (Softening of Water)
Hardness of water can be removed by certain methods.
- Temporary hardnesscan be removed by boiling. When boiled, soluble bicarbonates of calcium and magnesium turn into insoluble carbonates, which can be removed by filtration.
- The reaction for calcium bicarbonate is:

- The reaction for magnesium bicarbonate is:

- The reaction for calcium bicarbonate is:
- Permanent hardness can be removed by adding chemicals like washing soda (sodium carbonate). Washing soda turns soluble calcium sulphate and magnesium sulphate into insoluble carbonates, which can be removed by filtration.
- The reaction for calcium sulphate is:
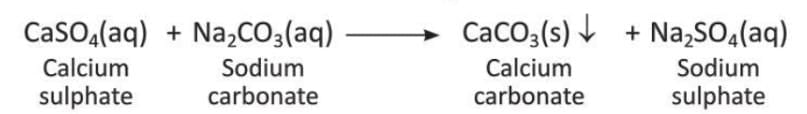
- The reaction for magnesium sulphate is:
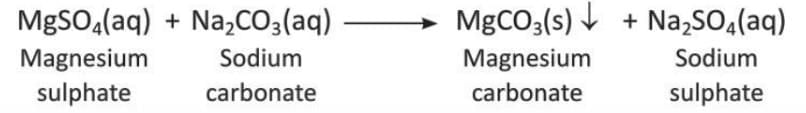
- The reaction for calcium sulphate is:
Glossary
- Water is very important for the survival of all living organisms.
- The main sources of water are rainwater, surface water, and groundwater.
- Rainwater is the purest form of water.
- Surface water consists of water present in oceans, seas, rivers, lakes, etc.
- Water cycle maintains the water balance between land, water bodies, and atmosphere; it also controls weather patterns and sustains life on Earth.
- Potable water should be clear, transparent, colorless, odorless, and free from dissolved and suspended impurities.
- The main processes involved in the purification of water from lakes or rivers are sedimentation, filtration, and chlorination.
- Purification of water at home is done by boiling, chlorination, and by using water filters and water purifiers.
- Pure water is colorless, transparent, odorless, and tasteless; its neutral pH is 7, it is highly stable.
- Pure water has a maximum density at 4°C and minimum density at 0°C; the lower density of ice at 0°C makes it lighter than water, so ice floats on water.
- Most substances contract when they are cooled; however, water expands when cooled; this is called anomalous expansion of water.
- Water is called a universal solvent because it dissolves many solids, liquids, and gases.
- Hardness of water is caused by dissolved chlorides, sulphates, and bicarbonates of calcium and magnesium.
- Hardness is of two types: temporary hardness, which contains bicarbonates of calcium and magnesium, and permanent hardness, which contains chlorides and sulphates of calcium and magnesium.
- Temporary hardness can be removed by boiling, and permanent hardness can be removed by adding chemicals such as washing soda (sodium carbonate).
Glossary
- Groundwater: Rainwater that gets collected under the ground.
- Water table: The level of water under the ground.
- Water cycle: The continuous circulation of water between land, water bodies, and atmosphere.
- Potable water: Water which is fit for consumption.
- Purification of water: The process of removal of undesirable chemicals, impurities, and biological contaminants from water.
- Boiling point: The constant temperature at which water boils and changes into steam.
- Freezing point: The constant temperature at which water freezes and changes into ice.
- Distillation: The substance that dissolves in water or any other liquid.
- Solute: Water or any other liquid in which solute dissolves.
- Solvent: The liquid mixture which results from the dissolution of a solute in a solvent.
- Solution: The water that easily forms lather with soap.
- Soft water: The water that does not form lather with soap.
- Hard water: The water that does not form lather with soap.
|
2 videos|64 docs|19 tests
|
FAQs on Water Chapter Notes - Chemistry for SSS 2
| 1. What are the physical properties of water? |  |
| 2. What is the difference between a true solution, suspension, and colloid? |  |
| 3. What is meant by water of crystallization? |  |
| 4. What is the difference between hard water and soft water? |  |
| 5. How does water's chemical properties affect its role in reactions? |  |