Chemical Equations | AP Chemistry - Grade 9 PDF Download
Writing Word Equations & Symbol Equations
Word equations
- Word equations depict reactants and products of a chemical reaction using their full chemical names.
- The arrow signifies the conversion of reactants into products; it can be read as "goes to" or "produces".
- Above the arrow, you may include reaction conditions or the name of a catalyst.
- For instance, a word equation for neutralization could be:
sodium hydroxide + hydrochloric acid → sodium chloride + water - Reactants: sodium hydroxide and hydrochloric acid
- Products: sodium chloride and water
Names of compounds
Chemical Naming Conventions- When two elements, such as hydrogen and chlorine, combine, the name of the compound is derived by adding "-ide" to the second element. For instance, the combination of hydrogen and chlorine is known as hydrogen chloride.
- For combinations of non-metals, the element with the lower group number is named first. For example, carbon and oxygen form CO2, known as carbon dioxide since carbon belongs to Group 4 and oxygen to Group 6.
Common Groups of Atoms
- Common groups of atoms, like carbonate, sulfate, hydroxide, and nitrate ions, are frequently encountered in chemistry. These groups have specific naming patterns when combined with metals.
Chemical Ions and Compounds
- Chemical ions like carbonate (CO32-), sulfate (SO42-), hydroxide (OH-), and nitrate (NO3-) are examples of charged particles.
- When these ions combine with a metal, the metal name precedes the ion name. For instance, KOH is potassium hydroxide, and CaCO3 is calcium carbonate.
Writing and Balancing Chemical Equations
- Chemical equations represent reactions using symbols for reactants and products. Balancing equations ensures the same number of atoms of each element on both sides.
- Non-metals like H2, N2, O2, F2, Cl2, Br2, and I2 exist as diatomic molecules.
- When balancing, move across the equation element by element. For unchanged groups like nitrate (NO3-), consider them as single entities.
- Examples of chemical equations:
- Acid-base neutralization: NaOH(aq) + HCl(aq) → NaCl(aq) + H2O(l)
- Redox reaction: 2Fe2O3(s) + 3C(s) → 4Fe(s) + 3CO2(g)
Chemical Equations
- Acid-base Neutralisation Reaction: Sodium hydroxide (NaOH) (aqueous) reacts with Hydrochloric acid (HCl) (aqueous) to form Sodium chloride (NaCl) (aqueous) and Water (H2O) (liquid).
- Redox Reaction: Two molecules of Iron(III) oxide (Fe2O3) (solid) react with three molecules of Carbon (C) (solid) to yield four molecules of Iron (Fe) (solid) and three molecules of Carbon dioxide (CO2) (gas).
- In each chemical equation, there is a balance of the same number of each atom on both sides of the reaction arrow, ensuring the equations are balanced.
Methods for Balancing Equations
- The best approach is to practice a lot of examples of balancing equations.
- By trial and error, change the coefficients (multipliers) in front of the formulas, one by one, checking the results on the other side.
- Balance elements that appear on their own, last in the process.
Chemical Equations and State Symbols
- Chemical reactions involve the transformation of substances into new compounds through rearrangement of atoms.
- Equations represent these reactions, with reactants on the left side and products on the right.
- State symbols are crucial in showing the physical state of each substance involved in a chemical equation.
State Symbols in Chemical Equations
- State symbols are written after each formula to indicate the physical state of the substance.
- Common state symbols include (s) for solid, (l) for liquid, (g) for gas, and (aq) for aqueous (dissolved in water).
Understanding State Symbols
- State symbols help us visualize how substances exist in a reaction.
- For example, (s) denotes a solid state, (l) represents a liquid, (g) signifies a gas, and (aq) indicates a substance dissolved in water.
Importance of State Symbols
- State symbols are essential for writing balanced chemical equations.
- They clarify the physical state of each substance, aiding in a better understanding of the reaction.
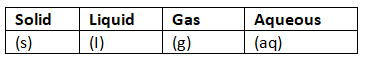
Symbol Equations in Chemical Reactions
- Symbol equations are crucial in chemical equations to represent reactions accurately.
- An illustration of this is the reaction between copper carbonate and hydrochloric acid:
CuCO3 (s) + 2HCl (aq) → CuCl2 (aq) + CO2 (g) + H2O (l)
Understanding State Symbols
- Determining the correct state symbol can sometimes be challenging and requires attention to substance identities.
Here are some guidelines:
- Metal compounds typically exist as solids, with a few exceptions.
- Ionic compounds are usually in solid form.
- Non-metal compounds can be solids, liquids, or gases based on their chemical structure.
- Precipitates formed in solutions are considered solids.
In practical examples, equations with state symbols would look like:
- 2Al (s) + 3CuO (s) → Al2O3 (s) + 3Cu (s)
- MgO (s) + 2HNO3 (aq) → Mg(NO3)2 (aq) + H2O (l)
State Symbols for Solutions
- When indicating the state of solutions, be cautious. For instance, ethanol is commonly a liquid, so if it's pure alcohol, use (l) as the state symbol. However, if alcohol is in a water solution, then (aq) is the appropriate symbol.
Deducing Symbol Equations
- For certain reactions, the unbalanced equation may not be provided. Instead, you are expected to utilize your acquired knowledge from the course to determine or infer the formulas of compounds and then proceed to balance the equations.
Example Scenario
- Consider the reaction where aluminum reacts with chlorine to produce the white solid, aluminum chloride. Your task is to formulate the balanced symbol equation, incorporating state symbols, for this particular chemical reaction.
Solution Approach
- Step 1: Begin by identifying the formulas and state symbols of the reactants and products to establish an unbalanced symbol equation:
Detailed Steps
- Aluminum, being a solid metal, has a chemical symbol that is identical to its formula: Al (s). Knowing that chlorine, a Group VII element, exists as a diatomic gas: Cl2 (g).
- Aluminum chloride, a solid compound, and its formula can be deduced from the charges on the ions involved: Aluminum carries a 3+ charge, while chloride ions carry a 1- charge. To maintain neutrality, 3 chloride ions combine with every 1 aluminum ion: AlCl3 (s).
- Therefore, the unbalanced symbol equation can be represented as:
- Properties of Aluminium: Aluminium is a solid metal and, like other pure metals, it is an element with the chemical symbol Al (s).
- Properties of Chlorine: Chlorine is a gas that exists as a diatomic molecule with the formula Cl2 (g).
- Properties of Aluminum Chloride:
- Aluminum chloride is a solid compound. Its formula, AlCl3 (s), is deduced from the charges on the ions present.
- Aluminium carries a 3+ charge, while chloride ions have a 1- charge. To maintain neutrality, 3 chloride ions are required for every 1 aluminium ion in the compound.
- Symbol Equation for Aluminum Chloride Formation: Al (s) + Cl2 (g) → AlCl3 (s)
- Steps to Balance the Equation:
- Make the number of Cl atoms on the right-hand side (RHS) even by adding a coefficient of 2 in front of AlCl3.
Al (s) + Cl2 (g) → 2 AlCl3 (s)
- Make the number of Cl atoms on the right-hand side (RHS) even by adding a coefficient of 2 in front of AlCl3.
Balancing Chemical Equations
- When balancing chemical equations, ensure that the number of each type of atom is the same on both sides.
- For instance, in the reaction: Al (s) + Cl2 (g) → AlCl3 (s), we balance the equation as follows:
- This gives 6 Cl on the RHS, so add a coefficient of 3 in front of Cl2 on the LHS:
- Finally, there are now 2 Al on the RHS but only 1 on the LHS, so add a coefficient of 2 in front of Al on the LHS:
2 Al (s) + 3 Cl2 (g) → 2 AlCl3 (s)
Balancing Ionic Equations
- In aqueous solutions, ionic compounds dissociate into their ions.
- For example, hydrochloric acid (HCl) dissociates into H+ and Cl- ions:
HCl (aq) → H+ (aq) + Cl- (aq)
Recognizing Common Ionic Compounds
It's crucial to identify common ionic compounds and their constituent ions. These compounds include:
- Acids like HCl and H2SO4
- Group I and Group II hydroxides, for instance, sodium hydroxide
- Soluble salts such as potassium sulfate and sodium chloride
Solved Example
Example: Write the ionic equation for the reaction of aqueous chlorine and aqueous potassium iodide.
Ans: Step 1: Write out the full balanced equation:
2KI (aq) + Cl2 (aq) → 2KCl (aq) + I2 (aq)
Step 2: Identify the ionic substances and write down the ions separately
2K+ (aq) + 2I- (aq) + Cl2 (aq) → 2K+ (aq) + 2Cl- (aq) + I2 (aq)
Step 3: Rewrite the equation eliminating the ions which appear on both sides of the equation (spectator ions ) which in this case are the K+ ions:
2I- (aq) + Cl2 (aq) → 2Cl- (aq) + I2 (aq)
Edurev Tip: When balancing equations, it's imperative not to alter any of the formulas themselves; only the quantities of each atom or molecule can be adjusted. This is achieved by modifying the coefficients placed in front of each chemical species.
|
71 videos|98 docs|40 tests
|
FAQs on Chemical Equations - AP Chemistry - Grade 9
| 1. What is the difference between a word equation and a symbol equation in chemistry? |  |
| 2. How can you deduce symbol equations from word equations in chemistry? |  |
| 3. What is the importance of writing balanced chemical equations in chemistry? |  |
| 4. How can you balance a chemical equation? |  |
| 5. Why is it important to understand chemical equations in Year 11 chemistry? |  |















