Class 7 Science Chapter 5 HOTS Questions - Physical and Chemical Changes
Q1: Explain how painting of an iron gate prevents it from rusting.
Ans: For rusting, the presence of both oxygen and water (or water vapour) is essential. Painting of an iron gate prevents it from coming in contact with oxygen, or water, or both and thus prevents it from rusting.
Q2: When we keep a piece of iron in the open area for few days, a brownish, flaky substance, called rust, is deposited on it.
(a) Is rust different from iron?
(b) Is formation of rust from iron a chemical change?
(c) Can you change rust back into iron by some simple method?
(c) Give some other examples of a similar type of changes.
Ans:
(a) Yes. Rust is oxide of iron.
(b) Yes, it is a chemical change as properties of iron has changed.
(c) No.
(d) (i)Setting of curd into milk (ii) Burning of magnesium ribbon in air (iii) Cooking of food.
Q3: Why lime water turns milky on passing carbon dioxide gas into it?
Ans: Chemical formula of lime water is calcium hydroxide which is a colourless solution. When we pass carbon dioxide gas into lime water, it forms white coloured insoluble calcium carbonate.
Lime water + Carbon dioxide ➝ Calcium carbonate + Water
Q4: Why stretching of rubber band is a physical change?
Ans: When rubber band is stretched only its size changes and it comes back in its original shape and size, once it is released. Moreover, it does not cause any change in its chemical composition. Hence, stretching of rubber band is a physical change.
Q5: How can you say that ripening of a fruit is a chemical change?
Ans: Ripening of a fruit is a chemical change because after ripening, a new product with different properties is formed.
Q6: Complete the following reaction
Ca (OH)2 + CO2 →
Ans:
Q7: Magnesium ribbon bums in air and changes to white substance, i.e. magnesium oxide. When magnesium oxide dissolves in water, what type of change take place? Give reason in support of your answer. Express the change in the form of equation.
Ans: Mixing of ash obtained by the burning of magnesium with water is a chemical change. When magnesium is burnt in air, it forms magnesium oxide in the form of white ash.
Magnesium (Mg)+ Oxygen (O2) → Magnesium oxide (MgO)
When magnesium oxide dissolves in water, it forms a new substance, magnesium hydroxide.
Magnesium oxide (MgO) + Water (H2O) → Magnesium hydroxide Mg(OH)2
So, it is a chemical change.
Q8: Plants prepare their food by a process called photosynthesis. Can we call photosynthesis is a chemical change? Explain.
Ans: During photosynthesis, the plants intake carbon dioxide and water in the presence of chlorophyll and sunlight to form two new substances, i.e. glucose (food) and oxygen gas. So, photosynthesis is a chemical change.
Q9: What happens when an iron blade of a knife is dipped in a copper sulphate solution? What kind of change takes place?
Ans: When an iron blade of a knife is dipped in a copper sulphate solution, then iron blade is coated with reddish brown deposits of copper. And the blue colour of copper sulphate solution changes to light green due to the formation of iron sulphate. So, it is a chemical change.
Q10: When baking soda is mixed with vinegar, bubbles are formed with the evolution of a gas. Name the gas evolved. What happens when this gas is passed through lime water?
Ans: When baking soda (sodium hydrogen carbonate) and vinegar (acetic acid) are mixed together, then a chemical change takes place between sodium hydrogen carbonate and acetic acid to form three new substances.
The change in the test tube is as follows:
Carbon dioxide gas produced in the reaction passing through freshly prepared lime water as shown in figure.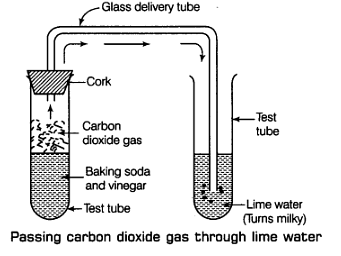
Lime water is calcium hydroxide solution. When carbon dioxide gas is passed through lime water, then calcium hydroxide combines with carbon dioxide to form a white solid substance, calcium carbonate which makes lime water milky. This chemical change can be written in the form of word equation as follows:

The reaction between lime water and carbon dioxide gas is a chemical change because a new substance calcium carbonate is formed during this change. The turning of lime water into milky is a standard test of carbon dioxide.
When baking soda (NaHC03) reacts with vinegar which contains acetic acid carbon dioxide comes out, which turns lime water milky, therefore it is a chemical change. In all these activities, we saw that in each change, one or more new substances are formed. When magnesium ribbon was burnt, the ash was the new substance formed.
The reaction of copper sulphate with iron produced two new substances, i.e. iron sulphate and copper. Vinegar and baking soda together produced carbon dioxide which turned lime water milky. So, all those changes in which one or more new substances formed, are called chemical changes. These are permanent changes which can usually not be reversed to form the original substance.
Q10: What happens when magnesium ribbon is burnt in air?
Ans: When the magnesium ribbon burnt in air, it reacts with oxygen found in the air to form Magnesium Oxide. After it burns, it forms a white powder of the magnesium oxide. The change can be represented by the following equation:
Magnesium (Mg) + Oxygen (O2) → Magnesium oxide (MgO)
Q11: Classify the following processes into physical or chemical changes.
(a) Beating of aluminium metal to make aluminium foil
(b) Digestion of food
(c) Cutting of a log of wood into pieces
(d) Burning of crackers
Ans: Physical changes are beating of aluminium metal to make aluminium foil and cutting of a log of wood into pieces. Chemical changes are digestion of food and burning of crackers.
Q12: Explain the following.
(a) Lime water turns milky on passing carbon dioxide gas through it.
(b) Bubbles are produced when acetic acid is added to a solution of sodium hydrogen carbonate.
Ans:
(a) Carbon dioxide gas produced in the reaction passing through freshly prepared lime water as shown in figure.
Lime water is calcium hydroxide solution. When carbon dioxide gas is passed through lime water, then calcium hydroxide combines with carbon dioxide to form a white solid substance, calcium carbonate which makes lime water milky. This chemical change can be written in the form of word equation as follows:

The reaction between lime water and carbon dioxide gas is a chemical change because a new substance calcium carbonate is formed during this change. The turning of lime water into milky is a standard test of carbon dioxide.
(b) When baking soda (sodium hydrogen carbonate) and vinegar (acetic acid) are mixed together, then a chemical change takes place between sodium hydrogen carbonate and acetic acid to form three new substances.
The change in the test tube is as follows:
Q13: Give an example of a chemical reaction for each of the following situations:
(a) A change in colour is observed.
(b) A gas is evolved.
(c) Sound is produced.
Ans:
(a) Chemical reaction between copper sulphate solution and iron metal. In this reaction, blue colour of copper sulphate solution changes to light green colour due to the formation of iron sulphate.

(b) When baking soda and vinegar are mixed together then a chemical change takes place and bubbles of carbon dioxide gas are formed along with some other substances.

(c) Explosion of a firework produces heat, light, sound and unpleasant gases. Explosion of a firework is a chemical change.
Q14: Which of the following is a physical change?
(a) Rusting of iron
(b) Combustion of magnesium ribbon
(c) Burning of candle
(d) Melting of wax
Ans: (d) Melting of wax
Q15: Which one of the following is a chemical change?
(a) Twinkling of stars
(b) Cooking of vegetables
(c) Cutting of fruits
(d) Boiling of water
Ans: (b) Cooking of vegetables
Q16: A man painted his main gate made up of iron, to
(i) prevent it from rusting.
(ii) protect it from the sun.
(iii) make it look beautiful.
(iv) make it dust free.
Which of the above statements is/are correct?
(a) (i) and (ii)
(b) (ii) and (iii)
(c) Only (ii)
(d) (i) and (iii)
Ans: (d) (i) and (iii)
Q17: Paheli’s mother made a concentrated sugar syrup by dissolving sugar in hot water. On cooling, crystals of sugar got separated.
This indicates a
(a) physical change that can be reversed
(b) chemical change that can be reversed
(c) physical change that cannot be reversed
(d) chemical change that cannot be reversed
Ans: (a) physical change that can be reversed
|
111 videos|246 docs|28 tests
|
FAQs on Class 7 Science Chapter 5 HOTS Questions - Physical and Chemical Changes
| 1. What are some examples of physical changes? |  |
| 2. Can physical changes be reversed? |  |
| 3. How can you differentiate between physical and chemical changes? |  |
| 4. What happens at the molecular level during a physical change? |  |
| 5. Is cutting paper a physical or chemical change? |  |

|
Explore Courses for Class 7 exam
|

|


















