Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action | Basic Physics for IIT JAM PDF Download
Cohesion and Adhesion in Liquids
Children blow soap bubbles and play in the spray of a sprinkler on a hot summer day. An underwater spider keeps his air supply in a shiny bubble he carries wrapped around him. A technician draws blood into a small-diameter tube just by touching it to a drop on a pricked finger. A premature infant struggles to inflate her lungs. What is the common thread? All these activities are dominated by the attractive forces between atoms and molecules in liquids—both within a liquid and between the liquid and its surroundings.
Attractive forces between molecules of the same type are called cohesive forces. Liquids can, for example, be held in open containers because cohesive forces hold the molecules together. Attractive forces between molecules of different types are called adhesive forces. Such forces cause liquid drops to cling to window panes, for example. In this section we examine effects directly attributable to cohesive and adhesive forces in liquids.
Cohesive Forces
Attractive forces between molecules of the same type are called cohesive forces.
Adhesive Forces
Attractive forces between molecules of different types are called adhesive forces.
The soap bubbles in this photograph are caused by cohesive forces among molecules in liquids.
Surface Tension
Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension. Molecules on the surface are pulled inward by cohesive forces, reducing the surface area. Molecules inside the liquid experience zero net force, since they have neighbors on all sides.
Surface Tension
Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension.
Making Connections: Surface Tension
Forces between atoms and molecules underlie the macroscopic effect called surface tension. These attractive forces pull the molecules closer together and tend to minimize the surface area. This is another example of a submicroscopic explanation for a macroscopic phenomenon.
The model of a liquid surface acting like a stretched elastic sheet can effectively explain surface tension effects. For example, some insects can walk on water (as opposed to floating in it) as we would walk on a trampoline—they dent the surface (a).(b) shows another example, where a needle rests on a water surface. The iron needle cannot, and does not, float, because its density is greater than that of water. Rather, its weight is supported by forces in the stretched surface that try to make the surface smaller or flatter. If the needle were placed point down on the surface, its weight acting on a smaller area would break the surface, and it would sink.
Surface tension supporting the weight of an insect and an iron needle, both of which rest on the surface without penetrating it. They are not floating; rather, they are supported by the surface of the liquid.
(a) An insect leg dents the water surface.  is a restoring force (surface tension) parallel to the surface.
is a restoring force (surface tension) parallel to the surface.
(b) An iron needle similarly dents a water surface until the restoring force (surface tension) grows to equal its weight.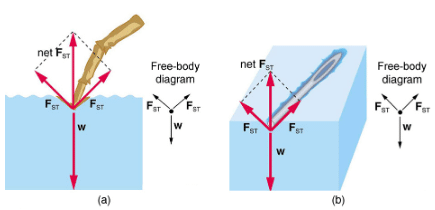
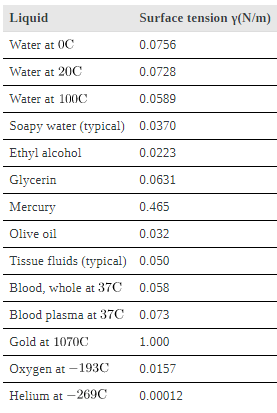
Surface tension is proportional to the strength of the cohesive force, which varies with the type of liquid. Surface tension γ is defined to be the force F per unit length L exerted by a stretched liquid membrane: γ = F/L.
Values of γ for some liquids. For the insect of (a), its weight w is supported by the upward components of the surface tension force: w=γLsinθ, where L is the circumference of the insect’s foot in contact with the water. One way to measure surface tension. The liquid film exerts a force on the movable wire in an attempt to reduce its surface area. The magnitude of this force depends on the surface tension of the liquid and can be measured accurately.
Surface tension is the reason why liquids form bubbles and droplets. The inward surface tension force causes bubbles to be approximately spherical and raises the pressure of the gas trapped inside relative to atmospheric pressure outside. It can be shown that the gauge pressure P inside a spherical bubble is given by P =4γ/r,
where r is the radius of the bubble. Thus the pressure inside a bubble is greatest when the bubble is the smallest. Another bit of evidence for this is illustrated. When air is allowed to flow between two balloons of unequal size, the smaller balloon tends to collapse, filling the larger balloon.
Sliding wire device used for measuring surface tension; the device exerts a force to reduce the film’s surface area. The force needed to hold the wire in place is F=γL=γ(2l), since there are two liquid surfaces attached to the wire. This force remains nearly constant as the film is stretched, until the film approaches its breaking point.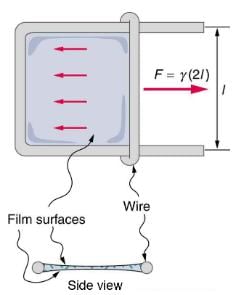
With the valve closed, two balloons of different sizes are attached to each end of a tube. Upon opening the valve, the smaller balloon decreases in size with the air moving to fill the larger balloon. The pressure in a spherical balloon is inversely proportional to its radius, so that the smaller balloon has a greater internal pressure than the larger balloon, resulting in this flow.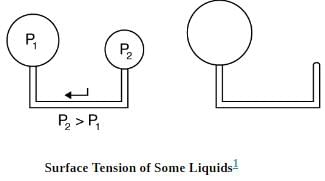
Surface Tension: Pressure Inside a Bubble
Calculate the gauge pressure inside a soap bubble 2.0010-4m in radius using the surface tension for soapy water. Convert this pressure to mm Hg.
Strategy
The radius is given and the surface tension, and so P can be found directly from the equation P =4γ/r.
Solution
Substituting r and γ into the equation P =4γ/r, we obtain
We use a conversion factor to get this into units of mm Hg:
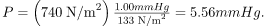
Discussion
Note that if a hole were to be made in the bubble, the air would be forced out, the bubble would decrease in radius, and the pressure inside would increase to atmospheric pressure (760 mm Hg).
Our lungs contain hundreds of millions of mucus-lined sacs called alveoli, which are very similar in size, and about 0.1 mm in diameter. You can exhale without muscle action by allowing surface tension to contract these sacs. Medical patients whose breathing is aided by a positive pressure respirator have air blown into the lungs, but are generally allowed to exhale on their own. Even if there is paralysis, surface tension in the alveoli will expel air from the lungs. Since pressure increases as the radii of the alveoli decrease, an occasional deep cleansing breath is needed to fully reinflate the alveoli. Respirators are programmed to do this and we find it natural, as do our companion dogs and cats, to take a cleansing breath before settling into a nap.
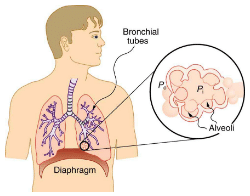
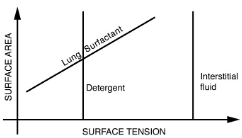
The tension in the walls of the alveoli results from the membrane tissue and a liquid on the walls of the alveoli containing a long lipoprotein that acts as a surfactant (a surface-tension reducing substance). The need for the surfactant results from the tendency of small alveoli to collapse and the air to fill into the larger alveoli making them even larger (as demonstrated). During inhalation, the lipoprotein molecules are pulled apart and the wall tension increases as the radius increases (increased surface tension). During exhalation, the molecules slide back together and the surface tension decreases, helping to prevent a collapse of the alveoli. The surfactant therefore serves to change the wall tension so that small alveoli don’t collapse and large alveoli are prevented from expanding too much. This tension change is a unique property of these surfactants, and is not shared by detergents (which simply lower surface tension).
Surface tension as a function of surface area. The surface tension for lung surfactant decreases with decreasing area. This ensures that small alveoli don’t collapse and large alveoli are not able to over expand.
If water gets into the lungs, the surface tension is too great and you cannot inhale. This is a severe problem in resuscitating drowning victims. A similar problem occurs in newborn infants who are born without this surfactant—their lungs are very difficult to inflate. This condition is known as hyaline membrane disease and is a leading cause of death for infants, particularly in premature births. Some success has been achieved in treating hyaline membrane disease by spraying a surfactant into the infant’s breathing passages. Emphysema produces the opposite problem with alveoli. Alveolar walls of emphysema victims deteriorate, and the sacs combine to form larger sacs. Because pressure produced by surface tension decreases with increasing radius, these larger sacs produce smaller pressure, reducing the ability of emphysema victims to exhale. A common test for emphysema is to measure the pressure and volume of air that can be exhaled.
Making Connections: Take-Home Investigation
(1) Try floating a sewing needle on water. In order for this activity to work, the needle needs to be very clean as even the oil from your fingers can be sufficient to affect the surface properties of the needle.
(2) Place the bristles of a paint brush into water. Pull the brush out and notice that for a short while, the bristles will stick together. The surface tension of the water surrounding the bristles is sufficient to hold the bristles together. As the bristles dry out, the surface tension effect dissipates.
(3) Place a loop of thread on the surface of still water in such a way that all of the thread is in contact with the water. Note the shape of the loop. Now place a drop of detergent into the middle of the loop. What happens to the shape of the loop? Why?
(4) Sprinkle pepper onto the surface of water. Add a drop of detergent. What happens? Why?
(5) Float two matches parallel to each other and add a drop of detergent between them. What happens? Note: For each new experiment, the water needs to be replaced and the bowl washed to free it of any residual detergent.
Adhesion and Capillary Action
Contact Angle
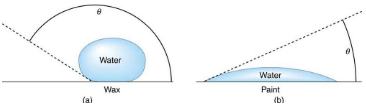
The angle θ between the tangent to the liquid surface and the surface is called the contact angle.
In the photograph, water beads on the waxed car paint and flattens on the unwaxed paint.
(a) Water forms beads on the waxed surface because the cohesive forces responsible for surface tension are larger than the adhesive forces, which tend to flatten the drop.
(b) Water beads on bare paint are flattened considerably because the adhesive forces between water and paint are strong, overcoming surface tension. The contact angle θ is directly related to the relative strengths of the cohesive and adhesive forces. The larger θ is, the larger the ratio of cohesive to adhesive forces.

One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary tube. This action causes blood to be drawn into a small-diameter tube when the tube touches a drop.
Capillary Action
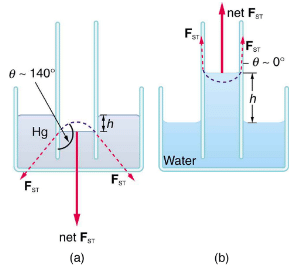
If a capillary tube is placed vertically into a liquid, capillary action will raise or suppress the liquid inside the tube depending on the combination of substances. The actual effect depends on the relative strength of the cohesive and adhesive forces and, thus, the contact angle θ given in the table. If θ is less than 90, then the fluid will be raised; if θ is greater than 90, it will be suppressed. Mercury, for example, has a very large surface tension and a large contact angle with glass. When placed in a tube, the surface of a column of mercury curves downward, somewhat like a drop. The curved surface of a fluid in a tube is called a meniscus. The tendency of surface tension is always to reduce the surface area. Surface tension thus flattens the curved liquid surface in a capillary tube. This results in a downward force in mercury and an upward force in water.
(a) Mercury is suppressed in a glass tube because its contact angle is greater than 90. Surface tension exerts a downward force as it flattens the mercury, suppressing it in the tube. The dashed line shows the shape the mercury surface would have without the flattening effect of surface tension.
(b) Water is raised in a glass tube because its contact angle is nearly θ. Surface tension therefore exerts an upward force when it flattens the surface to reduce its area.
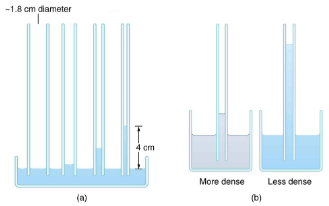
Capillary action can move liquids horizontally over very large distances, but the height to which it can raise or suppress a liquid in a tube is limited by its weight. It can be shown that this height h is given by h=2γcosθ/ρgr.
If we look at the different factors in this expression, we might see how it makes good sense. The height is directly proportional to the surface tension γ, which is its direct cause. Furthermore, the height is inversely proportional to tube radius—the smaller the radius r, the higher the fluid can be raised, since a smaller tube holds less mass. The height is also inversely proportional to fluid density ρ, since a larger density means a greater mass in the same volume.
(a) Capillary action depends on the radius of a tube. The smaller the tube, the greater the height reached. The height is negligible for large-radius tubes.
(b) A denser fluid in the same tube rises to a smaller height, all other factors being the same.
Calculating Radius of a Capillary Tube: Capillary Action: Tree Sap
Can capillary action be solely responsible for sap rising in trees? To answer this question, calculate the radius of a capillary tube that would raise sap 100 m to the top of a giant redwood, assuming that sap’s density is 1050kg/m3, its contact angle is zero, and its surface tension is the same as that of water at 20.0C.
Strategy
The height to which a liquid will rise as a result of capillary action is given by h=2γcosθ/ρgr, and every quantity is known except for r.
Solution
Solving for r and substituting known values produces
Discussion
This result is unreasonable. Sap in trees moves through the xylem, which forms tubes with radii as small as 2.510-5m . This value is about 180 times as large as the radius found necessary here to raise sap 100m. This means that capillary action alone cannot be solely responsible for sap getting to the tops of trees.
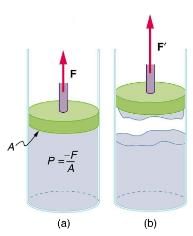
How does sap get to the tops of tall trees? (Recall that a column of water can only rise to a height of 10 m when there is a vacuum at the top.) The question has not been completely resolved, but it appears that it is pulled up like a chain held together by cohesive forces. As each molecule of sap enters a leaf and evaporates (a process called transpiration), the entire chain is pulled up a notch. So a negative pressure created by water evaporation must be present to pull the sap up through the xylem vessels. In most situations, fluids can push but can exert only negligible pull, because the cohesive forces seem to be too small to hold the molecules tightly together. But in this case, the cohesive force of water molecules provides a very strong pull. One device for studying negative pressure. Some experiments have demonstrated that negative pressures sufficient to pull sap to the tops of the tallest trees can be achieved.
(a) When the piston is raised, it stretches the liquid slightly, putting it under tension and creating a negative absolute pressure P = -F/A.
(b) The liquid eventually separates, giving an experimental limit to negative pressure in this liquid.
Section Summary
1. Attractive forces between molecules of the same type are called cohesive forces.
2. Attractive forces between molecules of different types are called adhesive forces.
3. Cohesive forces between molecules cause the surface of a liquid to contract to the smallest possible surface area. This general effect is called surface tension.
4. Capillary action is the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary tube which is due to the relative strength of cohesive and adhesive forces.
Conceptual Questions
The density of oil is less than that of water, yet a loaded oil tanker sits lower in the water than an empty one. Why?
Is surface tension due to cohesive or adhesive forces, or both?
Is capillary action due to cohesive or adhesive forces, or both?
Birds such as ducks, geese, and swans have greater densities than water, yet they are able to sit on its surface. Explain this ability, noting that water does not wet their feathers and that they cannot sit on soapy water.
Water beads up on an oily sunbather, but not on her neighbor, whose skin is not oiled. Explain in terms of cohesive and adhesive forces.
Could capillary action be used to move fluids in a “weightless” environment, such as in an orbiting space probe?
What effect does capillary action have on the reading of a manometer with uniform diameter? Explain your answer.
Pressure between the inside chest wall and the outside of the lungs normally remains negative. Explain how pressure inside the lungs can become positive (to cause exhalation) without muscle action.
Problems & Exercises
What is the pressure inside an alveolus having a radius of 2.5010-4-m if the surface tension of the fluid-lined wall is the same as for soapy water? You may assume the pressure is the same as that created by a spherical bubble.
592 N/m2
(a) The pressure inside an alveolus with a 2.0010-4-m radius is 1.40103 Pa, due to its fluid-lined walls. Assuming the alveolus acts like a spherical bubble, what is the surface tension of the fluid?
(b) Identify the likely fluid. (You may need to extrapolate between values.)
2.2310-2mm Hg
Calculate the force on the slide wire if it is 3.50 cm long and the fluid is ethyl alcohol
(a) shows the effect of tube radius on the height to which capillary action can raise a fluid.
(a) Calculate the height h for water in a glass tube with a radius of 0.900 cm—a rather large tube like the one on the left.
(b) What is the radius of the glass tube on the right if it raises water to 4.00 cm?
(a) 1.6510-3m
(b) 3.7110-4m
We stated that a xylem tube is of radius 2.5010-5m. Verify that such a tube raises sap less than a meter by finding h for it, making the same assumptions that sap’s density is 1050 kg/m3, its contact angle is zero, and its surface tension is the same as that of water at 20.0C.
What fluid is in the device if the force is 3.1610-3N and the length of the wire is 2.50 cm? Calculate the surface tension γ and find a likely match.
6.3210-2N/m
If the gauge pressure inside a rubber balloon with a 10.0-cm radius is 1.50 cm of water, what is the effective surface tension of the balloon?
Calculate the gauge pressures inside 2.00-cm-radius bubbles of water, alcohol, and soapy water. Which liquid forms the most stable bubbles, neglecting any effects of evaporation?
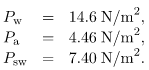
Alcohol forms the most stable bubble, since the absolute pressure inside is closest to atmospheric pressure.
Suppose water is raised by capillary action to a height of 5.00 cm in a glass tube.
(a) To what height will it be raised in a paraffin tube of the same radius?
(b) In a silver tube of the same radius?
Calculate the contact angle θ for olive oil if capillary action raises it to a height of 7.07 cm in a glass tube with a radius of 0.100 mm. Is this value consistent with that for most organic liquids?
5.1
This is near the value of θ=0 for most organic liquids.
When two soap bubbles touch, the larger is inflated by the smaller until they form a single bubble.
(a) What is the gauge pressure inside a soap bubble with a 1.50-cm radius?
(b) Inside a 4.00-cm-radius soap bubble?
(c) Inside the single bubble they form if no air is lost when they touch?
-2.78
What is the ratio of heights to which ethyl alcohol and water are raised by capillary action in the same glass tube?
Footnotes
1. 1 At 20ºC unless otherwise stated.
|
217 videos|156 docs|94 tests
|
FAQs on Cohesion and Adhesion in Liquids: Surface Tension and Capillary Action - Basic Physics for IIT JAM
| 1. What is cohesion and adhesion in liquids? |  |
| 2. How does surface tension work? |  |
| 3. What is capillary action and how does it relate to cohesion and adhesion? |  |
| 4. How does surface tension affect insects that can walk on water? |  |
| 5. Can surface tension be changed or modified? |  |
















