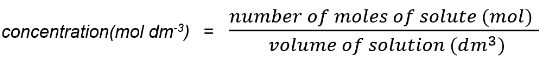Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Concentrations in mol/dm3
Concentrations in mol/dm3 | Chemistry for Grade 10 PDF Download
Concentration of Solutions in Moles
Higher Tier Only- It is more useful to a chemist to express concentration in terms of moles per unit volume rather than mass per unit volume
- Concentration can therfore be expressed in moles per decimetre cubed
- We can modify the concentration formula to include moles
- The units in the answer can be written as mol dm-3 or mol / dm3:

- The units in the answer can be written as mol dm-3 or mol / dm3:
- You may have to convert from g dm-3 into mol dm-3 and vice versa depending on the question
- To go from g dm-3 to mol dm-3:
- Divide by the molar mass in grams
- To go from mol dm-3 to g dm-3:
- Multiply by the molar mass in grams
- To go from g dm-3 to mol dm-3:
Exam Tip
Don't forget your unit conversions:
- To go from cm3 to dm3 : divide by 1000
- To go from dm3 to cm3 : multiply by 1000
Calculating Concentration from Reacting Solutions
- Solving problems on concentrations involves carefully working out moles and volumes in the correct units and applying the concentration formula
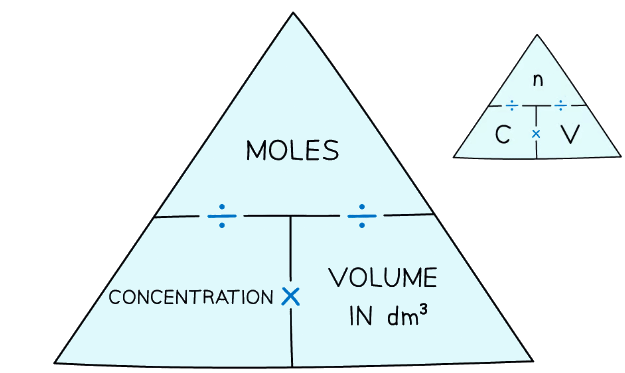
- Some students find formula triangles help them to understand the relaitonship:
 The concentration-moles formula triangle can help you solve these problems
The concentration-moles formula triangle can help you solve these problems - The following examples show how to do this step-by-step
Solved Examples
Example 1: Calculate the amount of solute, in moles, present in 2.5 dm3 of a solution whose concentration is 0.2 mol dm-3.Example 2: Calculate the concentration of a solution of sodium hydroxide, NaOH, in mol dm-3, when 80 g is dissolved in 500 cm3 of water.(Na= 23, H= 1, O= 16)
Titration Calculations
- If the concentration of one of the reactants is known (either the acid or the base), then the exact volumes from a titration along with the balanced chemical equation for the reaction can be used to calculate the concentration of the other reactant
Example 3: 25.0 cm3 of a solution of 0.05 mol dm-3 sodium carbonate was completely neutralised by 20.00 cm3 of dilute hydrochloric acid. Calculate the concentration of the hydrochloric acid in mol dm-3.
Exam Tip
You are not given the concentration-moles formula triangle in exams so you have to learn it. It is a good idea to write it down before you start a problem, so you get all the parts in the correct place.
The document Concentrations in mol/dm3 | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
78 videos|87 docs|11 tests
|
Related Searches