Conformational Analysis of Acyclic & Cyclic Systems | Organic Chemistry PDF Download
Conformational analysis : The different spat ial arrangements o f atoms in a mo lecule which are readily inter convert ible by rotation about single bonds are called conformations; if not conformation. The term conformation and configuration are related to energy barrier for Interconversion of different spatial arrangements of atoms in a molecule.
(i) For conformat ion energy barrier for conversio n of different spat ial arrangements is >0.6 and <16 kcal/mole (ii) For configuration energy barrier for conversion of different spatial arrangements is ≥16 kcal/mole.
The study if the existence of preferred conformations in a molecules and the relating of physical and chemical properties of a molecule to its preferred conformation is known as conformational analysis.
Conformation of acyclic systems
Staggered conformation:-
conformation with a dihedral angle of 600 is known as staggered conformation. The angle between the atoms attached to the front and the rear carbon atoms is called torsional angle.
Eclipsed conformation:- A conformation with a 00 torsional angle is known as eclipsed conformation.
(A) Conformation of ethane
When an ethane molecule rotates about its carbon-carbon single bond, two extreme conformations can result
1. Staggered conformation
2. Eclipsed conformation
An infinite number of conformations between these two conformations are also possible.
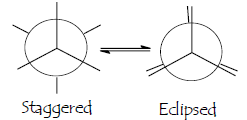
The electron in a C-H bond will repel the electrons in another C-H bond if the bonds get too close to each
other. Therefore, the staggered conformation is the most stable conformation because the C-H bonds are as far away from each other as possible. The eclipsed conformation is the least stable because the C—H bonds are closest. The distance between hydrogen nuclei is 2.55 A0 in staggered and 2.29 A0 in eclipsed conformation.
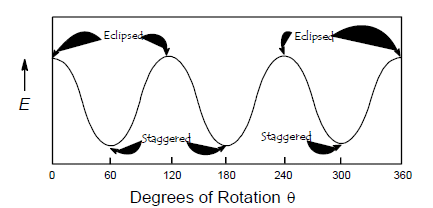
The rotational energy barrier in ethane is 2.9 kcal. The extra energy of the eclipsed conformation is due to the repulsion felt by bonding electrons of one substituent as they close to the bonding electrons of the other substituent. The energy barrier for ethane is 2.9 kcal which is more than the free energy for rotation i.e. ~0.6 kcal and less than restricted rotation i.e. 16 kcal at room temperature. So at the room temperature we can’t get the conformation of ethane.
The different spatial arrangements of atoms in a molecule which are readily inter convertible by rotation about single bonds are called conformations; if not conformation. The term conformation and configuration are related to energy barrier for Interconversion of different spatial arrangements of atoms in a molecule.
(i) For conformation energy barrier for conversion of different spatial arrangements is >0.6 and <16 kcal/mole
(ii) For configuration energy barrier for conversion of different spatial arrangements is ≥16 kcal/mole.
The study if the existence of preferred conformations in a molecules and the relating of physical and chemical properties of a molecule to its preferred conformation is known as conformational analysis.
- Torsional Strain: Extra energy of eclipsed conformation arising due to the repulsion between bonding electrons of one substituent with that of the other as they pass close to each other
- Steric Strain: Strain induced when two atoms or groups in a molecule are too close to each other, arising due to repulsion between electron cloud of interacting atoms/groups.
- Angle Strain: Strain induced in molecules when the bond angles are different from the desired tetrahedral bond angle of 109.5°
(B) Conformation of n-butane
Butane may be treated as a derivative of ethane where one hydrogen on each carbon is replaced by a methyl group. The conformation of butane will be symmetrical only if the rotation will be about C2—C3 bond.
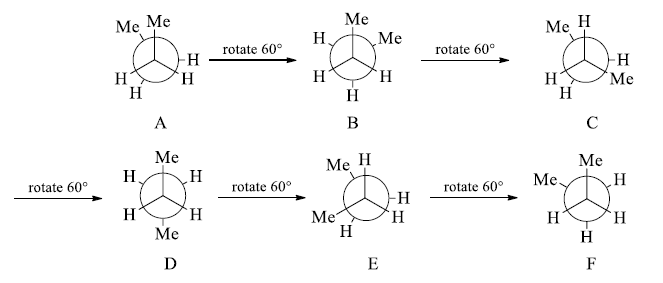
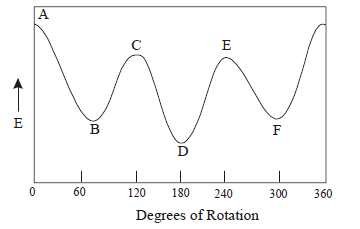
Butane has three conformations which are staggered (B, D, F). (D) in which the two methyl groups are as far apart as possible is more stable than the other two staggered conformations (B) and (D). The most stable of the staggered is called anti conformation and other two conformations are called gauche conformation.
In the anti conformation the larger substituents are opposite to each other, in the gauche conformer, they are adjacent. The two gauche conformers have the same energy barrier but each is 0.9 kcal/mole less stable than anti conformation.
Anti and gauche conformers do not have the same energy because of steric strain. Steric strain is the strain put on a molecule when its atoms or groups are large in size and due to this they are too close to each other, which cause repulsion between the atoms or groups.
All other eclipsed conformation has torsional and steric strain. The (A) conformation is much unstable because the two methyl groups eclipsed each other and cause much steric strain.
Anti(D) > gauche (B, F) > eclipsed (C, E) > fully eclipsed (A).
Conformation of cyclic systems
A. Cyclopropanes
Propane: Angle and steric strains are absent (all angles are nearly tetrahedral). It is fully staggered conformer).
Cyclopropane: It has deviation of 49.5° per carbon plus torsional strain: (C-H) … (C-H) eclipsing interactions.
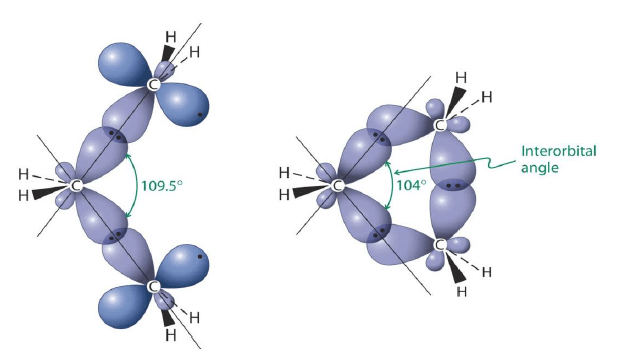
Cyclopropane suffers from angle strain and torsional strain. Strain relief through the formation of“banana” bonds. Renders higher reactivity, Weaker C-C and C-H bonds.
(C) Cyclobutane
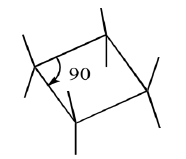
Planar structure has angular strain and torsional strain. So compromise is ring puckering. Relieves eclipsing interactions with only a slight increase in angle strain (90 to 88°). Hence, Cyclobutane has a ‘wing shaped or ‘puckered’conformer.
B. Cyclopentane
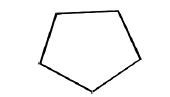
Planar? Though the angle strain is very little, there will be 10 eclipsing interactions!
Ring puckering relieves eclipsing interactions with only a slight increase in angle strain. It has four coplanar carbon atoms. Cyclopentane has an ‘envelope’ conformer.
D. Cyclohexane
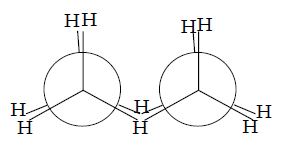
Chair conformation of cyclohexane
This conformation of cyclohexane resembles a chair and hence called chair form.
1. This conformation is free from angle strain.
2. The close resemblance of the chair conformation of cyclohexane with the staggered conformation of ethane can be well appreciated from the following diagrams.
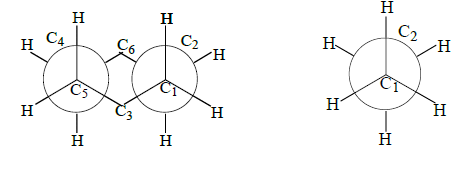
Resemblance between chair conformation of cyclohexane and ethane in staggered form.
1. The chair form of cyclohexane represents the staggered conformation and hence it is free from torsional strain.
2. The chair form, therefore lies at the energy minimum and is the most stable conformation of cyclohexane and of almost all its derivatives.
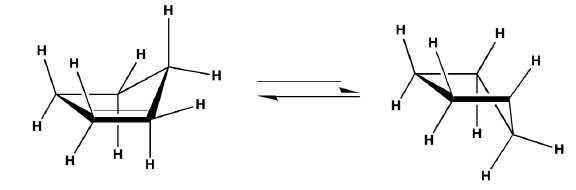
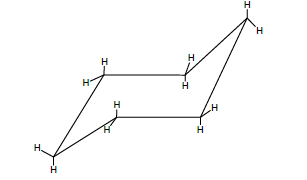
Boat conformation of cyclohexane
If we flip up the lower end of the model of the chair form we will get the boat conformation
1. It is obvious that these results from rotation about single bonds, hence we are indeed dealing with the conformation of cyclohexane.
2. If we sight the model of the boat conformation from the front side, we find the hydrogen’s on two sets of C—C bonds in perfectly eclipsed conformation
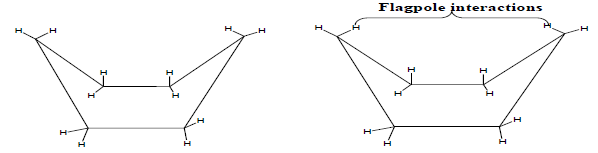
Twist Boat conformation of cyclohexane
1. If we twist the two flagpole hydrogen’s in the model of the boat conformation of cyclohexane in the opposite direction a new conformation results, which is known as twist-boat conformation.

2. The hydrogen’s on two sets of C—C bonds are not in perfectly eclipsed positions, which relieves the torsional-strain considerably. The distance between flagpole hydrogen’s also increases, as a result of which the Vander Waals repulsion between them decreases. The twist boat conformation is therefore more stable than the boat conformation.
Half-chair conformation of cyclohexane
1. If we flip the lower end of chair form then new conformation results known as half chair. In it H atoms of adjacent carbon atoms are fully eclipsed.
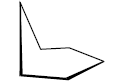
2. In half chair there is angular strain. So, half chair conformation is less stable.
Stability order:-
Chair > Half chair > Twist boat > Boat
Conformational free energy
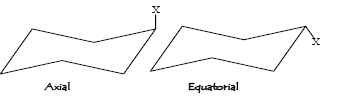
The two diastereomeric chair forms are of unequal energy and so are differently populated, the equilibrium constant K being given by the equation:-
ΔG0=-RT lnK
ΔG0 is the difference of free energy between the Equatorial and axial conformers and -ΔG0 is known as conformational free energy of the substituent. (Sometimes known as A value).
It determines the equatorial preference of the substituent in the substituted cyclohexane. The conformational free energies (-ΔG0 values) of a number of common substituent are given below-
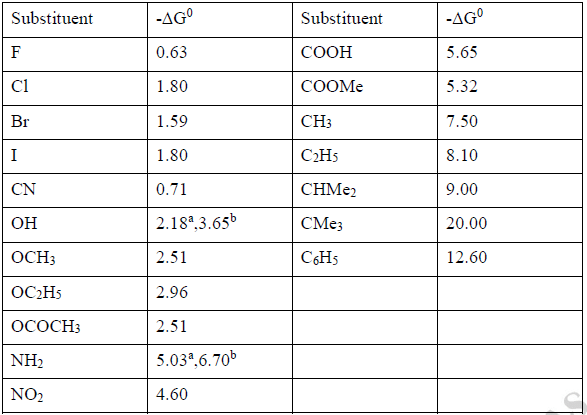
*a=aprotic solvent; b= protic solvent.
|
44 videos|102 docs|52 tests
|
FAQs on Conformational Analysis of Acyclic & Cyclic Systems - Organic Chemistry
| 1. What is conformational analysis? |  |
| 2. What are acyclic systems in conformational analysis? |  |
| 3. How is conformational analysis performed for cyclic systems? |  |
| 4. What are the factors influencing the stability of conformations in conformational analysis? |  |
| 5. How is conformational analysis useful in drug design and synthesis? |  |

















