Cram’s rule and Prelog Rule | Organic Chemistry PDF Download
During certain additions to the carbon-oxygen double bond of aldehydes and ketones containing an asymmetric α carbon cram’s rule predicts diastereoselectivity i.e. which diastereomer will predominate. According to this rule the incoming nucleophilic group preferentially attacks on the side of the plane containing the small group..
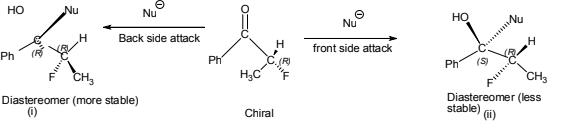
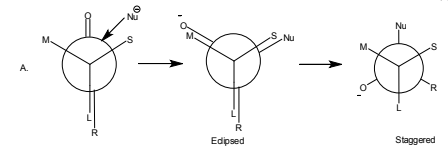
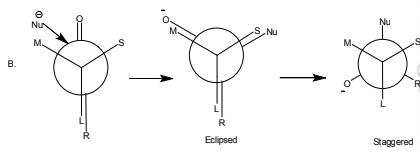
By this rule, it can be predicted that the reaction(A) should preferred over (B).
When the size of incoming group increases the extent of diastereoselectivity also increases. As in the above shown example if we increases the size of incoming group i.e. nucleophile from Me<Et<Ph then ratio of the major product formed is also increased.
Prelog rule
This rule is applied for α-keto esters. This reaction is enantioselective as one enantiomer is preffered which is formed by attack on α-carbonyl carbon from less hindered site.
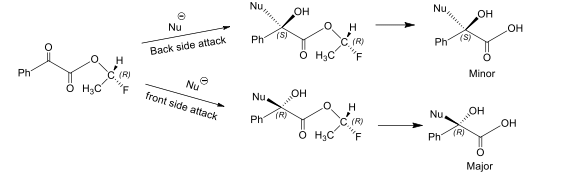
From the above reaction two compounds are formed which are non-superimposable mirror images of each other i.e. enantiomers. According to this rule, incoming nucleophile will attack from that side of the plane which contains group of lowest priority.
Topic relation ship of ligands and faces
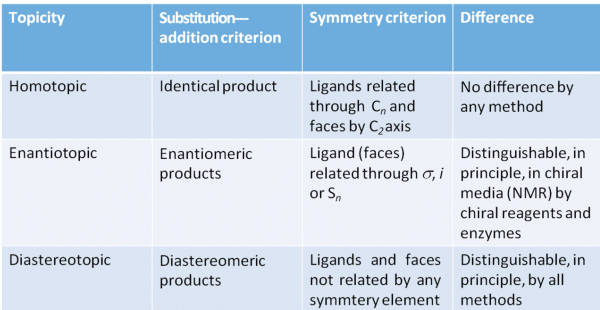
|
44 videos|102 docs|52 tests
|
FAQs on Cram’s rule and Prelog Rule - Organic Chemistry
| 1. What is Cram's rule and how is it used in chemistry? |  |
| 2. How does Cram's rule apply to determining the major product in reactions involving cyclic compounds? |  |
| 3. What is the Prelog Rule and how is it used in stereochemistry? |  |
| 4. How does the Prelog Rule help in determining the configuration of chiral centers? |  |
| 5. Can Cram's rule and the Prelog Rule be applied together in organic chemistry? |  |

















