Electrolysis of Aqueous Solutions | Chemistry for Grade 10 PDF Download
| Table of contents |

|
| Electrolysis of an Aqueous Solution Using Inert Electrodes |

|
| Electrode Reactions |

|
| Solved Examples |

|
| Determining what Gas is Produced |

|
Electrolysis of an Aqueous Solution Using Inert Electrodes
- Aqueous solutions will always contain water molecules (H2O)
- In the electrolysis of aqueous solutions, the water molecules dissociate producing H+ and OH– ions:
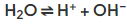
- These ions are also involved in the electrolysis process and their chemistry must be considered
We now have an electrolyte that contains ions from the compound plus ions from the water
Which ions get discharged and at which electrode depends on the relative reactivity of the elements involved
Note: The concentration of the solution can affect the products of electrolysis, however, this is beyond the scope of this course and you are not expected to know the specific details of this
Exam Tip
When answering questions on this topic, it helps if you first write down all of the ions present first. Only then you should start comparing their reactivity and deducing the products formed.
Electrode Reactions
Positive Electrode (anode)
- Negatively charged OH– ions and non-metal ions are attracted to the positive electrode
- If halide ions (Cl-, Br-, I-) and OH- are present then the halide ion is discharged at the anode, gains electrons and forms a halogen (chlorine, bromine or iodine)
- If no halide ions are present, then OH- is discharged at the anode, gains electrons and forms oxygen
- In both cases the other negative ion remains in solution
Negative Electrode (cathode)
- Positively charged H+ and metal ions are attracted to the negative electrode but only one will gain electrons
- Either hydrogen gas or the metal will be produced
- If the metal is above hydrogen in the reactivity series, then hydrogen will be produced and bubbling will be seen at the cathode
- This is because the more reactive ions will remain in solution, causing the least reactive ion to be discharged
- Therefore at the cathode, hydrogen gas will be produced unless the positive ions from the ionic compound are less reactive than hydrogen, in which case the metal is produced
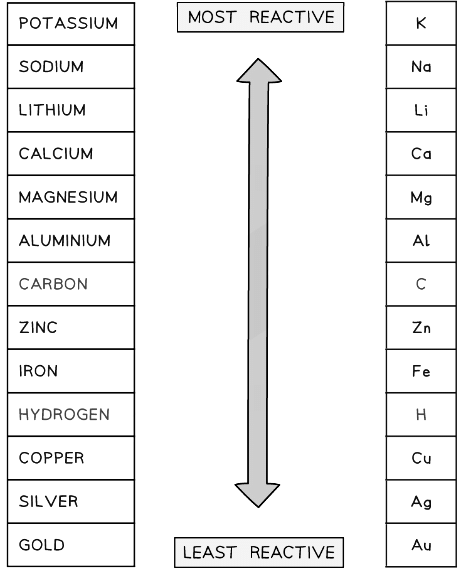 The reactivity series of metals including hydrogen and carbon
The reactivity series of metals including hydrogen and carbon
Solved Examples
Example 1: Predict the products formed at each electrode in the electrolysis of magnesium iodide solution
- Work out what ions are attracted to the cathode
- Hydrogen (H+) and magnesium (Mg2+)
- Decide which element will be discharged:
- The less reactive element is usually formed
- Hydrogen is discharged at the cathode as it is less reactive than magnesium
- Work out what ions are attracted to the anode
- Hydroxide (OH-) and iodide (I-)
- Decide which element will be discharged:
- If a halide is present, the corresponding halogen is formed, otherwise, oxygen is formed
- Iodine is discharged at the anode as iodide ions are present
Example 2: Predict the products formed at each electrode in the electrolysis of copper sulfate solution
- Work out what ions are attracted to the cathode
- Hydrogen (H+) and copper (Cu2+)
- Decide which element will be discharged:
- The less reactive element is usually formed
- Copper is discharged at the cathode as it is less reactive than hydrogen
- Work out what ions are attracted to the anode
- Hydroxide (OH-) and sulfate (SO42-)
- Decide which element will be discharged:
- If a halide is present, the corresponding halogen is formed, otherwise, oxygen is formed
- Oxygen is discharged at the anode as no halide ions are present
Determining what Gas is Produced
- The gas produced can be tested to determine its identity
- If the gas produced at the cathode burns with a ‘pop’ with a lighted splint then the gas is hydrogen
- If the gas produced at the anode relights a glowing splint dipped into the gas then the gas is oxygen
- If the gas produced at the anode turns damp blue litmus paper red and is then bleached white then the gas is chlorine
- The halogen gases all produce their own colours (bromine is red-brown, chlorine is yellow-green)
Exam Tip
Once you have identified the ions, the next step is to decide towards which electrode will they be drawn and identify the product formed. It helps if you recall the reactivity series.
|
75 videos|131 docs|24 tests
|




















