Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Electrolysis to Extract Metals
Electrolysis to Extract Metals | Chemistry for Grade 10 PDF Download
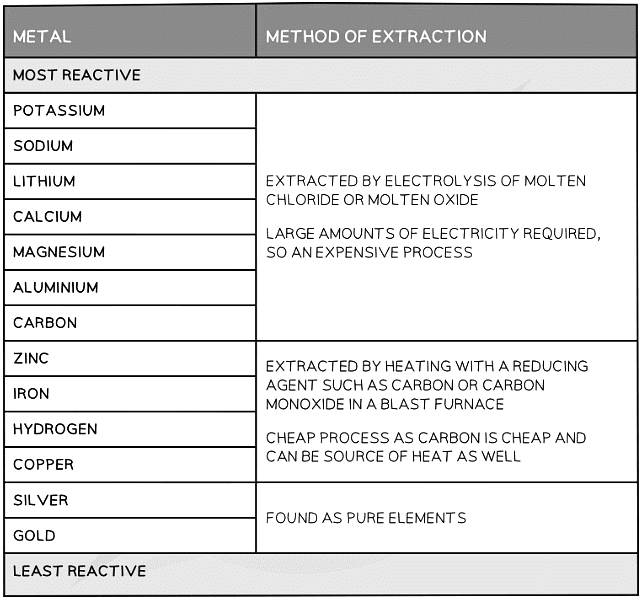
Choosing the Method of Extraction
- The position of the metal on the reactivity series determines the method of extraction
- Higher placed metals (above carbon) have to be extracted using electrolysis as they are too reactive and cannot be reduced by carbon
- Lower placed metals can be extracted by heating with carbon which reduces
- Electrolysis is very expensive as large amounts of energy are required to melt the ores and produce the electrical current
- The reactivity series of metals is shown below with the corresponding method of extraction
Metal Extraction Methods Summary Table
Exam Tip
Questions on this topic often ask you to explain why in some cases electrolysis is used and in other cases reduction by heating with carbon is used. Make sure you can explain when each process is used and why.
The Extraction of Aluminium
- Aluminium is extracted on a large scale using electrolysis:
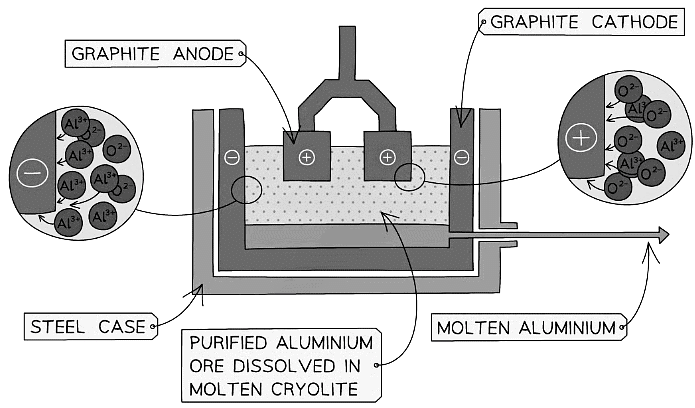 Diagram showing the extraction of aluminium by electrolysis
Diagram showing the extraction of aluminium by electrolysis
Raw Materials
- Aluminium Ore (bauxite)
- Cryolite (sodium aluminium fluoride)
Explanation:
- The bauxite is first purified to produce aluminium oxide, Al2O3
- Aluminium oxide has a very high melting point so it is first dissolved in molten cryolite producing an electrolyte with a lower melting point, as well as a better conductor of electricity than molten aluminium oxide
- This reduces the costs considerably making the process more efficient
- The electrolyte is a solution of aluminium oxide in molten cryolite at a temperature of about 1000 °C
- The molten aluminium is siphoned off from time to time and fresh aluminium oxide is added to the cell
- The cell operates at 5-6 volts and with a current of 100,000 amps
- The heat generated by the huge current keeps the electrolyte molten
- A lot of electricity is required for this process of extraction which is a major expense
- The overall equation is:
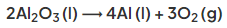
- Some of the oxygen produced at the positive electrode then reacts with the graphite (carbon) electrode to produce carbon dioxide gas:
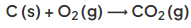
- This causes the carbon anodes to burn away, so they must be replaced regularly
Exam Tip
Questions on this topic often ask you to explain why cryolite is used in the process so make sure you are able to explain its use, providing reasons for your answer.
The document Electrolysis to Extract Metals | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
82 videos|200 docs|26 tests
|
Related Searches




















