Electrolysis | General Awareness for SSC CGL PDF Download
| Table of contents |

|
| Introduction |

|
| Batteries |

|
| Fuel Cells |

|
| Corrosion |

|
| Catalysis |

|
| Enzyme Catalysis |

|
Introduction
Electrolysis is conducted in an electrolytic cell, where an external voltage source induces a chemical reaction. A basic electrolytic cell includes two copper strips immersed in an aqueous copper sulfate solution. When DC voltage is applied to the electrodes, copper metal is deposited on the cathode, and copper dissolves at the anode.
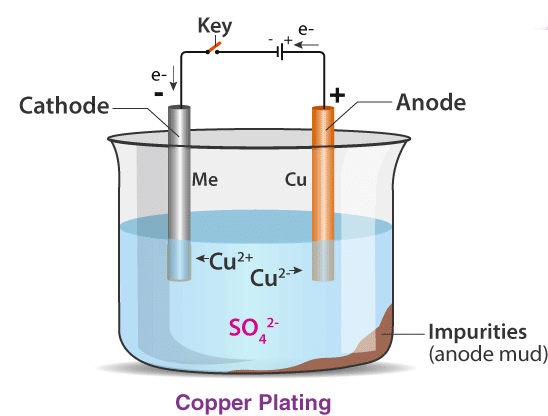
Applications of Electrolysis:
- Purification of Metals: For instance, impure copper is refined into high-purity copper through electrolytic purification.
- Extraction of Metals: Metals like sodium and magnesium are obtained by electrolyzing their fused chlorides, and aluminum is produced by electrolyzing molten aluminum oxide with cryolite.
- Organic Compound Preparation: Electrolysis aids in synthesizing organic compounds.
- Typographic Industries: Electrolysis is used to prepare blocks for printing.
- Galvanization: Steel is coated with zinc during galvanization.
- Electrochemical Series: Metals are arranged in increasing order of their standard reduction electrode potentials to form the electrochemical series:
K < Ca < Na < Mg < Al < Zn < Cr < Fe < Ni < H < Cu < Hg < Ag < Pd < Pt < Au. - Electrochemical Cells: An electrochemical cell converts chemical energy into electrical energy through a spontaneous redox reaction. Batteries, which convert chemical energy into electrical energy, are widely used in various devices and instruments. A battery should be lightweight, compact, and maintain consistent voltage during use. There are two main types of batteries: primary and secondary.
Batteries
- Batteries convert stored chemical energy into electrical energy.
- They are commonly utilized in various devices and tools.
- A good battery should be lightweight and compact.
- Its voltage should remain stable throughout its use.
- There are two primary categories of batteries: primary and secondary.
Primary Batteries
In primary batteries, the reaction occurs only once, and the battery eventually becomes dead.
- Dry Cell (Leclanche Cell): Consists of a zinc container (anode) and a carbon rod surrounded by manganese dioxide and carbon (cathode). The electrolyte is a moist paste of ammonium chloride and zinc chloride. Commonly used in transistors and clocks.
- Mercury Cell: Used in low-current devices like hearing aids and watches. It has a zinc-mercury amalgam anode and a cathode made of mercuric oxide and carbon paste. The electrolyte is a paste of potassium hydroxide and zinc oxide.
Secondary Batteries
Secondary batteries can be recharged by passing current through them in the opposite direction.
- Lead Storage Battery: Contains a lead anode and a lead dioxide-packed grid as the cathode, with a 38% sulfuric acid solution as the electrolyte. On charging, lead sulfate is converted back to lead and lead dioxide.
- Nickel Cadmium Cell: Has a longer life than the lead storage cell, with a cadmium anode and nickel dioxide cathode, and a potassium hydroxide solution as the electrolyte.
Fuel Cells
Fuel cells directly convert the energy from fuel combustion (e.g., hydrogen, carbon monoxide, methane) into electrical energy. A hydrogen-oxygen fuel cell, used in the Apollo Space Program, bubbles H2 and O2 through porous carbon electrodes into a concentrated aqueous sodium hydroxide solution, with palladium or platinum catalysts enhancing electrode efficiency. Hydrogen-oxygen fuel cells are non-polluting, producing only water as a byproduct, and operate with an efficiency of 70-75%, providing continuous energy.
Microbial Fuel Cells (MFCs)
MFCs are bioelectrochemical devices used in water treatment to harvest energy through anaerobic digestion, collecting bioenergy from wastewater.
Corrosion
Many metals, such as iron, are easily corroded by air and water. Rust, a brown flaky substance, forms on iron exposed to moist air, primarily consisting of hydrated iron (III) oxide (Fe2O3·xH2O). Other examples of corrosion include the tarnishing of silver and the formation of green coatings on copper or bronze. Corrosion involves the oxidation of a metal by losing electrons to oxygen. Rusting of iron can be prevented by painting, oiling, greasing, galvanizing (zinc coating), or chrome plating.
Catalysis
A catalyst alters the rate of a reaction without being consumed in the process, remaining unchanged in mass and composition. Catalysis refers to the phenomena where a catalyst affects the reaction rate. A solid catalyst is typically more effective when finely divided. Catalysts do not initiate reactions or change the equilibrium state of reversible reactions; they merely speed up the process by lowering the activation energy.
Applications in Industrial Processes:
- Haber Process: Iron as a catalyst and molybdenum as a promoter for ammonia synthesis.
- Contact Process: Vanadium pentoxide for sulfuric acid production.
- Ostwald Process: Platinum gauze for nitric acid production.
- Deacon Process: Cupric chloride for chlorine production.
- Synthesis of Petrol: Nickel, iron, cobalt, and alumina as catalysts.
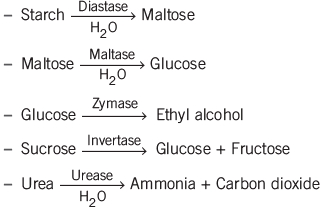
Enzyme Catalysis
Enzyme catalysis involves enzymes increasing the rate of reactions. Enzymes, which are proteins, are highly specific and sensitive to temperature, with an optimum range between 25-37°C. The rate of enzymatic reactions is significantly affected by pH changes.
|
528 videos|2108 docs|339 tests
|
FAQs on Electrolysis - General Awareness for SSC CGL
| 1. What is the difference between batteries and fuel cells? |  |
| 2. How does corrosion impact the efficiency of batteries and fuel cells? |  |
| 3. What role does catalysis play in the functioning of batteries and fuel cells? |  |
| 4. How does enzyme catalysis contribute to the development of more efficient batteries and fuel cells? |  |
| 5. How can electrolysis be used in the production of batteries and fuel cells? |  |















