Electrophiles & Nucleophiles | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| Electrophile: |

|
| Nucleophile: |

|
| Activators & deactivators |

|
| Heat of hydrogenation(H.O.H) |

|
Electrophile:
All electron-deficient atom or group of atoms are known as Electrophiles, the electrophile attacks at the electron-rich centre.
(a) all positively charged species are electrophiles.
H , NO2 , Br , Cl , etc.
(b) The compound in which the octet of central atom is not complete
BF3, AlCl3, ZnCl2, etc.
(c) all the compounds in which the central atom can expand its octet
SnCl4, SiCl4, etc.
(d) all polarising functional group are electrophiles as well as nuelophiles
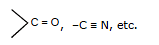

Nucleophile:
All electron rich compounds are nucleophiles and attack at the electron deficient centre.
(a) all negatively charged species
H-, Cl-, NO2-, Br-, CH3- etc.
(b) the compound in which the central atom has lone pair of electron.
NH3, H2O,  ,
,  etc.
etc.
(c) all organometallic compounds are nucleophiles
R - Mgx, RLi, R2Cd.
(d) The compound having p e- density, CH2 = CH2, etc.
Nucleophilicity:
The power of nucleophile is known as nucleophilicity .
→ The nucleophilicity of negative charge is greater than the nucleophilicity of lone pair


→ If lone pair or -ve charge is present on the different atoms then less electronegativity, more will be the nucleophilicity.
 ,
,  ,
,  ,
, 

→ NH3 < PH3 < AsH3 < SbH3 < BiH3 (Nucleophilicity)
→ If -ve charge or lone pair of electron is present on the same atom then the less stable -ve charge will be the better nucleophile

 (nucleophilicity)
(nucleophilicity)
Activators & deactivators
The groups in benzene which show M effect or I effect increases the electron density on benzene it means they activate the ring towards electrophile and known as activator.
 ,
,  , -CH3, -OR, -NHMe,
, -CH3, -OR, -NHMe,  ,
, 
The groups which shows -M or -I effect (resultant) decreases the e- density from benzene ring. It means they deactivate the ring towards electrophile.
 ,
,  ,
,  ,
,  ,
, 
 ,
,  ,
,  etc.
etc.
Ortho, Para & meta director:
The groups which shows +I (resultant) or +M effect then negative charge is developed at the ortho & para position. Therefore electrophile attack at the ortho & para position and the groups are known as OP director.
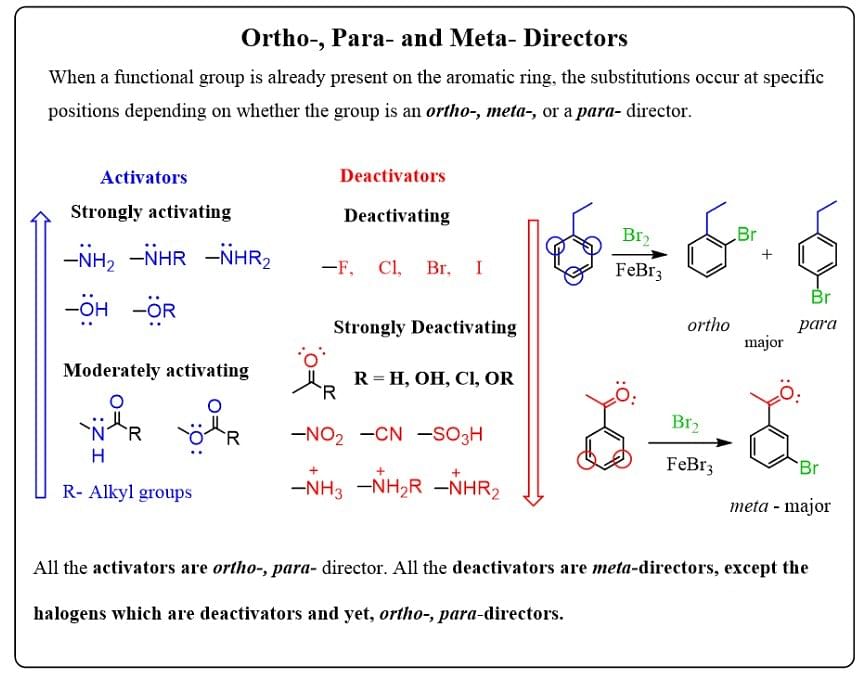

The groups which shows -M effect or - I effect (resultant) then -ve charge is developed at the ortho & para position this means electron density is minimum at the ortho & para positions and electrophile will attack at the meta position the groups are known as meta director.

 ,
,  ,
,  ,
,  ,
,  ,
,
 ,
,  ,
, 

Heat of hydrogenation(H.O.H)
It is the amount of energy released when one mole of H2 is added to any unsaturated system.
CH2 = CH2 + H2 → CH3 - CH3
HOH is exothermic process DH = - ve
*HOH ≈ No. of p-bonds in compound
If no. of p-bonds is same then
*HOH ≈ 
In case of alkene,
**
Ex. a 
b 
c 
b > c > a
Energy

Some examples of Aromatic(A), Non-aromatic(NA) and Anti-aromatic(AA)
It decides the stability of the compound and the heat of hydrogenation as well. Aromatic compounds are more stable and having less heat of Hydrogenation.
(1)  (A) (2)
(A) (2)  (AA) (3)
(AA) (3)  (AA)
(AA)
(4)  (NA) (5)
(NA) (5)  (AA) (6)
(AA) (6)  (A)
(A)
(7)  (A) (8)
(A) (8)  (A)
(A)
(9)  (A) (2pe) (10)
(A) (2pe) (10)  (A) (6pe) (11)
(A) (6pe) (11)  (NA)
(NA)
(12)  (NA) (13)
(NA) (13)  (AA) (4pe) (14)
(AA) (4pe) (14)  (A)
(A)
(15)  (NA) (16)
(NA) (16)  (A) (17)
(A) (17)  (A)
(A)
(18)  (AA) (19)
(AA) (19)  (A)
(A)
(20)  (AA) (21)
(AA) (21)  (AA)
(AA)
(22)  (AA) (23)
(AA) (23)  (A) (24)
(A) (24)  (A)
(A)
(25)  (A) (26)
(A) (26)  (A) (27)
(A) (27)  (NA)
(NA)
|
352 videos|596 docs|309 tests
|
FAQs on Electrophiles & Nucleophiles - Chemistry for JEE Main & Advanced
| 1. What is an electrophile? |  |
| 2. What is a nucleophile? |  |
| 3. What are activators and deactivators? |  |
| 4. How does heat of hydrogenation (H.O.H) relate to electrophiles and nucleophiles? |  |
| 5. What are some examples of electrophiles and nucleophiles? |  |
|
352 videos|596 docs|309 tests
|

|
Explore Courses for JEE exam
|

|


















