Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Energy Changes & Reversible Reactions
Energy Changes & Reversible Reactions | Chemistry for Grade 10 PDF Download
Reversible Reactions
- Some reactions go to completion, where the reactants are used up to form the product molecules and the reaction stops when the reactants have been exhausted
- In reversible reactions, the product molecules can themselves react with each other or decompose and form the reactant molecules again
- It is said that the reaction can occur in both directions: the forward reaction (which forms the products) and the reverse direction (which forms the reactants)
- When writing chemical equations for reversible reactions, two opposing arrows are used to indicate the forward and reverse reactions occurring at the same time
- Each one is drawn with just half an arrowhead – the top one points to the right, and the bottom one points to the left
- The direction a reversible reaction takes can be changed by changing the reaction conditions
- For example heating ammonium chloride produces ammonia and hydrogen chloride gases:

- As the hot gases cool down they recombine to form solid ammonium chloride
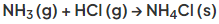
- So, the reversible reaction is represented like this:

Exam Tip
The reverse reaction may also be called the backwards reaction. A generic reversible reaction is shown as
A + B ⇌ C + D
Energy Changes & Reversible Reactions
- Energy changes also accompany chemical changes and energy can be given out (exothermic) or taken in (endothermic)
- The majority of chemical reactions are exothermic with only a small number being endothermic
- For a reversible reaction, if it is exothermic in one direction then it must be endothermic in the opposite direction
- The amount of energy transferred in either direction is the same
- Reversible reactions can be seen in some hydrated salts
- These are salts that contain water of crystallisation which affects their shape and colour
- Water of crystallisation is the water that is included in the structure of some salts during the crystallisation process
- A common example is copper(II) sulfate which crystallises forming the salt copper(II) sulfate pentahydrate, CuSO4.5H2O
- Water of crystallisation is indicated with a dot written in between the salt and the surrounding water molecules
- Anhydrous salts are those that have lost their water of crystallisation, usually by heating, in which the salt becomes dehydrated
- When anhydrous copper(II) sulfate is added to water, it turns blue and heat is given off so the reaction is exothermic
- When hydrated copper(II) sulfate crystals are heated in a test tube, the blue crystals turn into a white powder and a clear, colourless liquid (water) collects at the top of the test tube
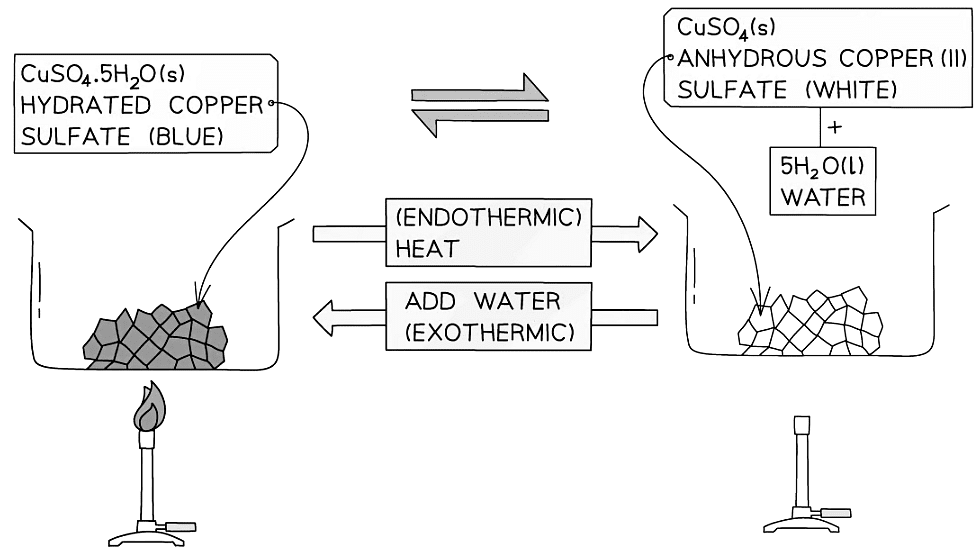 Energy changes & reversible reactions example
Energy changes & reversible reactions example
Exam Tip
Make sure you know the terms anhydrous, hydrated and water of crystallisation.
The document Energy Changes & Reversible Reactions | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches













