Environmental Chemistry | General Awareness for SSC CGL PDF Download
Introduction
The environment is the physical and biological world around us, consisting of three main components:
- Abiotic (Non-living) Components
- Biotic (Living) Components
- Energy Components
Abiotic Components
Abiotic components, or abiotic factors, are the non-living chemical and physical parts of the environment that affect ecosystems. These include the atmosphere, hydrosphere, and lithosphere.
Atmosphere: The atmosphere provides essential gases like oxygen, nitrogen, and carbon dioxide. Oxygen is necessary for respiration in plants and animals, while carbon dioxide is needed for photosynthesis. The atmosphere carries water vapor responsible for rain and shields life from harmful UV rays, maintaining the Earth's surface temperature. It is composed of several layers:
- Troposphere: The lowest layer, reaching up to 18 km, is a turbulent and dusty zone containing air (N2, O2, CO2), water vapor, and clouds.
- Stratosphere: Located 18-60 km above sea level, this layer contains the ozone layer (ozonosphere) between 20-40 km, which absorbs about 99.5% of harmful UV radiation. Since, O3 is unstable and again splits into O2 and O, a dynamic equilibrium exists between production and dissociation of ozone molecule.
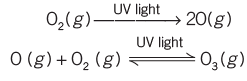
- Mesosphere: Extending from 60-85 km, temperatures here decrease with height, reaching -100°C. Meteors burn up in this layer.
- Thermosphere: Above the mesosphere, extending up to 640 km, temperatures can rise to 1500°C, but due to low pressure, it doesn't feel warm. The international space station orbits in this layer.
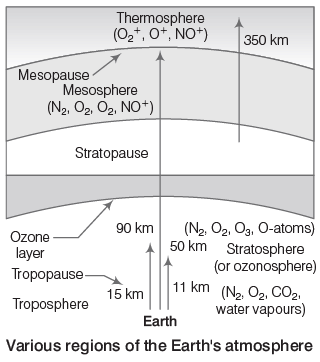
- Exosphere: The highest region, between 500-1600 km, contains ionized gases and leads to interstellar space.
Hydrosphere: Encompasses all water sources on Earth, such as oceans, rivers, lakes, and ponds. Ocean water, containing about 3.5% dissolved salts, is not suitable for drinking.
Lithosphere: The solid, rocky crust of the Earth, composed of minerals, covers the planet's surface from the top of Mount Everest to the bottom of the Mariana Trench.
Biosphere: The part of the lithosphere, hydrosphere, and atmosphere where living organisms reside and interact.
Biotic Components
Biotic components are the living organisms in an ecosystem. Biotic factors include any living component that affects another organism, such as animals that consume other organisms and the food that organisms consume. Each biotic factor requires energy for work and food for growth.
Atmospheric Pollution
Atmospheric pollution is the presence or addition of undesirable substances in the atmosphere, adversely affecting plants, animals, and humans. It can result from natural phenomena or human activities and is categorized into tropospheric and stratospheric pollution. Tropospheric pollution can be either gaseous or particulate. Pollutants, the substances causing pollution, are classified into:
- Primary Pollutants: Persist in the environment in their original form, e.g., sulfur dioxide (SO2) and nitrogen dioxide (NO2).
- Secondary Pollutants: Form from reactions involving primary pollutants, e.g., peroxyacetyl nitrate (PAN), ozone (O3), and aldehydes.
Pollutants from the Iron and Steel Industry
The iron and steel industry pollutes air, water, and soil, significantly impacting the environment. Air pollutants from this industry include CO2, SO2, NOx, CO, H2S, PAN, Pb, Ni, and Cd. Water pollutants include organic matter, oils, metals, acids, sulfides, and sulfates.
Major Gaseous Air Pollutants
Major gaseous air pollutants include oxides of sulfur, nitrogen, carbon, and hydrocarbons.
Sulfur Dioxide (SO2):
- Highly toxic to animals and plants, causing respiratory diseases like bronchitis, asthma, and emphysema, as well as eye and throat irritation.
- In plants, it reduces chloroplast formation, causing chlorosis.
- SO2 is corrosive, damaging buildings and textiles.
- It oxidizes to SO3 in the presence of particulate matter and NO2 or H2O, forming H2SO4, which contributes to acid rain.
Oxides of Nitrogen:
- Nitric oxide (NO) and nitrogen dioxide (NO2) act as tropospheric pollutants.
- NO2 is highly toxic to living tissues, causing leaf fall and retarding photosynthesis.
- It is a corrosive oxide that contributes to smog formation.
- NO2 causes eye irritation and can affect the liver and kidneys.
- It reacts with water to form nitric acid (HNO3), contributing to acid rain.
Carbon Monoxide (CO):
- Highly poisonous, it forms a stable carboxyhemoglobin complex with hemoglobin, blocking oxygen delivery to organs and tissues. This complex is about 200 times more stable than the oxygen-hemoglobin complex, reducing blood’s oxygen-carrying capacity, leading to headaches, nervousness, muscular weakness, weak eyesight, and asphyxia. Soil microorganisms act as a sink for CO.
Hydrocarbons:
- Consisting of only carbon and hydrogen, these are produced by the incomplete combustion of fuels in automobile engines and by anaerobic decomposition of organic matter.
- Methane (CH4) is the most abundant hydrocarbon pollutant.
- High concentrations of hydrocarbons are carcinogenic, causing aging in plants, breakdown of plant tissues, and leaf shedding.
- Hydrocarbons react with oxides of nitrogen to form secondary pollutants.
Prevention and Control of Air Pollution:
- Combustion: Converts organic pollutants into less harmful carbon dioxide (CO2) and water (H2O).
- Absorbers: Remove one or more pollutants from gaseous effluents.
- Fabric Filters: Trap and collect dust particles from gases, which are then discharged particle-free.
- Electrostatic Precipitators: Remove aerosols like dust, fumes, or mists from gases, precipitating the aerosol particles on electrodes and discharging particle-free gases.
Consequences of Atmospheric Pollution
 Global Warming and Greenhouse Effect
Global Warming and Greenhouse Effect
- The Earth's atmosphere allows most sunlight to pass through and heat the surface.
- Greenhouse gases such as carbon dioxide, methane, and water vapor trap the heat radiated from the Earth, leading to an increase in the Earth's temperature.
- This phenomenon is known as the greenhouse effect.
- The greenhouse effect is essential for the existence of life, as it prevents the Earth from becoming extremely cold.
- However, an increase in greenhouse gas concentrations intensifies the greenhouse effect, leading to global warming.
- Global warming may cause the melting of ice caps and glaciers, resulting in a rise in sea levels.
Acid Rain
- Acid rain is caused by the presence of oxides of nitrogen and sulfur in the air.
- These oxides dissolve in rainwater, forming nitric acid and sulfuric acid.
- The acidic rain then reacts with marble monuments (such as the Taj Mahal), buildings, and statues, leading to their gradual degradation.
- Acid rain also makes the soil acidic, reducing forest and agricultural productivity.
- Furthermore, acid rain dissolves heavy metals like lead, copper, mercury, and aluminum from soils, rocks, and sediments.
- These metal ions enter well water, causing various toxic effects.
- Lead, for instance, can cause anemia, brain damage, convulsions, and death.
- Other metals may affect the kidneys, liver, circulatory system, and nervous system.
- Many fungicides cause nerve damage and death, while toxic pesticides like DDT accumulate in our bodies, leading to kidney disorders and problems with the brain and circulatory system.
The Taj Mahal and Marble CancerIndia's Taj Mahal, located in Agra, is suffering from discoloration of its white marble due to air pollutants. Industries around Agra produce pollutants like sulfur dioxide and nitrogen dioxide, which cause acid rain. Acid rain corrodes the marble of the Taj Mahal, a phenomenon known as marble cancer. The Supreme Court of India has ordered industries to switch to cleaner fuels like CNG and LPG and mandated the use of unleaded petrol in the Taj zone to protect the monument.
Particulates
Particulates are minute solid particles and liquid droplets dispersed in the air, such as mists, dust, smoke, fumes, and bacteria.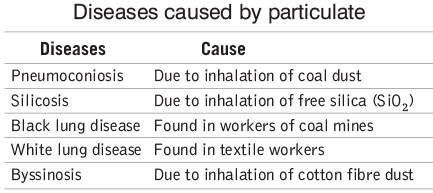
Smog
Smog is classified into two types:
- Classical Smog: Occurs in cool, humid climates. It is primarily composed of sulfur dioxide (SO2) and particulate matter from fuel combustion and is chemically reducing.
- Photochemical Smog: Occurs in warm, dry, and sunny climates. It consists of a mixture of primary pollutants (nitrogen oxides and carbon monoxide) and secondary pollutants (ozone and formaldehyde). Photochemical smog is common in cities with large populations and high vehicular density. It is an oxidizing smog, and ozone gives it a pungent smell and causes various respiratory diseases. Ozone also damages rubber articles. Peroxyacetyl nitrate (PAN) and aldehydes in smog cause eye irritation and have high toxicity to plants, affecting younger leaves and causing bronzing and glazing of their surfaces. Photochemical smog can be controlled by compounds acting as free radical traps, which react with the free radical precursors of smog when sprayed in the atmosphere. Efficient catalytic converters in automobiles also help prevent the release of nitrogen oxides and hydrocarbons into the atmosphere.
Stratospheric Pollution
- In the stratosphere, the ozone layer absorbs the Sun's ultraviolet radiation, which is harmful to living organisms.
- Depletion of the ozone layer leads to skin cancer and cataracts in humans, reduces plankton populations in oceans, and harms plants.
- Chlorofluorocarbons (CFCs), used in refrigeration, fire extinguishers, aerosol sprays, plastic foam production, tubeless tires, and electronic cleaning, cause ozone depletion.
- These compounds persist in the atmosphere for years and release chlorine atoms when decomposed by UV rays.
- One chlorine free radical can destroy a thousand ozone molecules.
- Ozone depletion, leading to the formation of an ozone hole, has been primarily observed in the Antarctic stratosphere.
- The ozone hole forms due to Polar Stratospheric Clouds (PSCs) and the inflow of CFCs and is most prominent during spring, after which it gets replenished.
Water Pollution
- Water pollution occurs due to foreign substances like sewage, algae, and soluble salts, known as water pollutants.
- In some parts of India, drinking water is contaminated with arsenic, fluoride, and uranium.
- Major water pollutants include pathogens (like bacteria), organic wastes (like leaves and grass), and chemical pollutants (like metals from industrial waste and petroleum).
- Dissolved Oxygen (DO) levels should be 5-6 ppm for healthy aquatic life; below 5 ppm, fish growth is inhibited.
- Biochemical Oxygen Demand (BOD) measures the oxygen required by microbes to decompose organic matter in water; Chemical Oxygen Demand (COD) measures the oxygen consumed by pollutants.
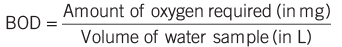
- Clean water has a BOD of less than 5 ppm; highly polluted water has a BOD of 17 ppm or more.
- The Ganga Action Plan, launched in 1985, aimed to reduce pollution in the river Ganga.
Soil Pollution
- Soil pollution refers to alterations in soil composition that reduce productivity due to undesirable substances like fertilizers and pesticides (positive soil pollution) or due to soil erosion and overuse (negative soil pollution).
- Landscape pollution occurs when fertile land becomes barren due to waste dumping.
- Pesticides (insecticides like DDT, herbicides like NaClO3, fungicides like organomercury compounds) and fertilizers are major sources of soil pollution.
- Soil conditioners (e.g., compounds of As, Hg, Pb) and solid wastes (industrial, farm, radioactive) also contribute to soil pollution.
Green chemistry focuses on processes and products that minimize the use and generation of hazardous substances.
Examples include:- Using carbon dioxide as a blowing agent in polystyrene foam manufacturing to eliminate ozone-depleting CFCs.
- Developing a safer marine antifouling compound, ‘sea-nine,’ that degrades faster than polluting organotins.
Strategy for Pollution Control
- Recycling: Converts waste into useful products, such as:
- Using scrap metal to manufacture steel.
- Recovering energy from burning combustible waste.
- Sewage Treatment: Controlling the dumping of sewage sludge or treating it before discharge into rivers or oceans.
- Incineration: Burns organic materials at over 1000°C in excess oxygen, converting them to harmless carbon dioxide and water, reducing waste volume.
- Digestion: Produces biogas and manure from biodegradable waste, which can treat sewage sludge.
- Oil Zapping: Uses bacteria to clean up oil spills, a bioremediation technique used on the Mumbai shoreline in August 2010.
Electronic Waste
- Electronic waste includes discarded computers, office equipment, entertainment electronics, mobile phones, TVs, and refrigerators.
- Computer components contain toxic substances like dioxins, PCBs, cadmium, chromium, radioactive isotopes, and mercury.
- A typical monitor may contain more than 6% lead by weight, primarily in the Cathode Ray Tube (CRT) glass.
|
478 videos|1421 docs|396 tests
|
FAQs on Environmental Chemistry - General Awareness for SSC CGL
| 1. What are some major gaseous air pollutants? |  |
| 2. What are the consequences of atmospheric pollution? |  |
| 3. How can stratospheric pollution impact the environment? |  |
| 4. What are some strategies for pollution control? |  |
| 5. What are some examples of abiotic components in an ecosystem? |  |
















