Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Extraction of Metals & Reduction
Extraction of Metals & Reduction | Chemistry for Grade 10 PDF Download
- The Earth’s crust contains metals and metal compounds such as gold, copper, iron oxide and aluminium oxide
- Useful metals are often chemically combined with other substances forming ores
- A metal ore is a rock that contains enough of the metal to make it worthwhile extracting
- They have to be extracted from their ores through processes such as electrolysis, using a blast furnace or by reacting with more reactive material
- In many cases the ore is an oxide of the metal, therefore the extraction of these metals is a reduction process since oxygen is being removed
- Common examples of oxide ores are iron and aluminium ores which are called haematite and bauxite respectively
- Unreactive metals do not have to be extracted chemically as they are often found as the uncombined element
- This occurs as they do not easily react with other substances due to their chemical stability
- They are known as native metals and examples include gold and platinum which can both be mined directly from the Earth’s crust.
Exam Tip
A metal can reduce another metal (remove oxygen) only if it is more reactive than the metal that is bonded to the oxygen.
Extraction of metals and the reactivity series
- The most reactive metals are at the top of the series
- The tendency to become oxidised is thus linked to how reactive a metal is and therefore its position on the reactivity series
- Metals higher up are therefore less resistant to oxidation than the metals placed lower down which are more resistant to oxidation
- The position of the metal on the reactivity series determines the method of extraction
- Higher placed metals (above carbon) have to be extracted from their compounds using electrolysis as they are too reactive and cannot be reduced by carbon
- Lower placed metals can be extracted from their compounds by heating with carbon which reduces them
- E.g. The oxides of metals which are below carbon can be reduced by heating them with carbon
- The carbon removes the oxygen from the metal oxide
- Carbon dioxide is formed as well as the metal element:
metal oxide + carbon → metal + carbon dioxide
- E.g. The oxides of metals which are below carbon can be reduced by heating them with carbon
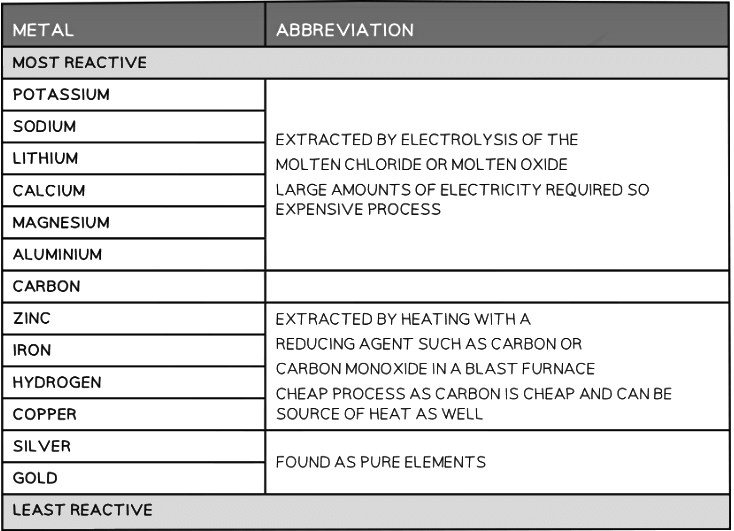
The extraction method depends on the position of a metal in the reactivity series
Exam Tip
Make sure you can explain why aluminium is extracted by electrolysis while iron is extracted by reduction as it is a question that often comes up.
The document Extraction of Metals & Reduction | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches




















