Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Flame Tests
Flame Tests | Chemistry for Grade 10 PDF Download
About Flame Tests
- Metal ions produce a colour if heated strongly in a flame
- Ions from different metals produce different colours
- The flame test is thus used to identify metal ions by the colour of the flame they produce
- Dip the loop of an unreactive metal wire such as nichrome or platinum in dilute acid, and then hold it in the blue flame of a Bunsen burner until there is no colour change
- This cleans the wire loop and avoids contamination
- This is an important step as the test will only work if there is just one type of ion present
- Two or more ions means the colours will mix, making identification erroneous
- Dip the loop into the solid sample and place it in the edge of the blue Bunsen flame
- Avoid letting the wire get so hot that it glows red otherwise this can be confused with a flame colour
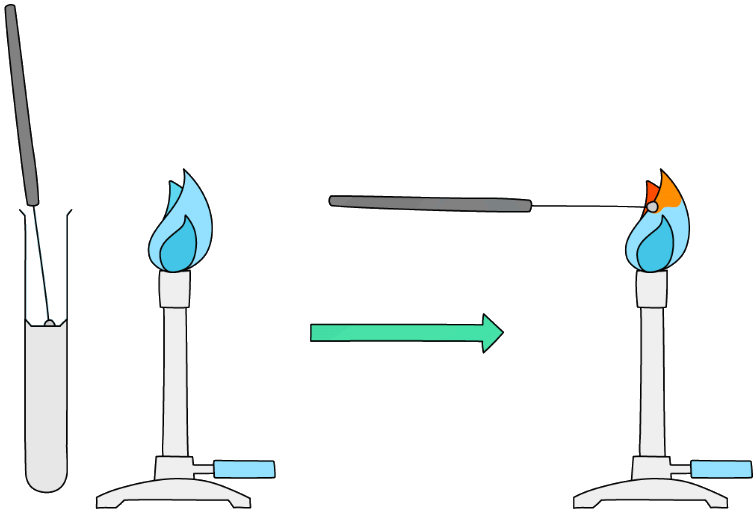 Diagram showing the technique for carrying out a flame test
Diagram showing the technique for carrying out a flame test
- The colour of the flame is observed and used to identify the metal ion present
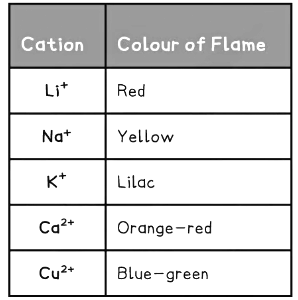
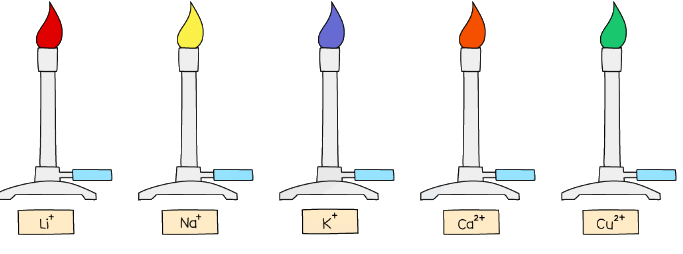 Diagram showing the colours formed in the flame test for metal ions
Diagram showing the colours formed in the flame test for metal ions
Exam Tip
The sample needs to be heated strongly, so the Bunsen burner flame should be on a blue flame.
The document Flame Tests | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches















