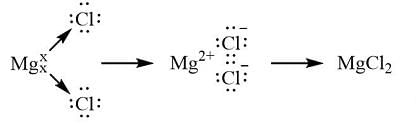|
True or False: The concept of atoms was experimentally validated by Maharishi Kanad and Pakudha Katyayama. |
Card: 3 / 40 |
|
False. Their ideas were philosophical and lacked experimental validation until later. |
Card: 4 / 40 |
|
He established the foundation of chemical sciences by formulating two important laws of chemical combination.  |
Card: 6 / 40 |
|
The Law of Conservation of Mass states that mass can neither be created nor destroyed in a chemical reaction. |
Card: 8 / 40 |
|
In a chemical substance, the elements are always present in ___ proportions by mass. |
Card: 9 / 40 |
|
True or False: According to John Dalton's Atomic Theory, all atoms of different elements are identical in mass and properties. |
Card: 11 / 40 |
|
He formulated the atomic theory, by explaining the Law of Conservation of Mass and the Law of Definite Proportions.  |
Card: 14 / 40 |
|
Fill in the blank: Atoms are the defining structure of an element and cannot be broken by ___ means. |
Card: 15 / 40 |
|
True or False: The International Union of Pure and Applied Chemistry (IUPAC) approves element names and symbols based solely on their historical origins. |
Card: 17 / 40 |
|
False. IUPAC approves element names and symbols based on standardized formats that ensure clarity and consistency, not just historical origins. |
Card: 18 / 40 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
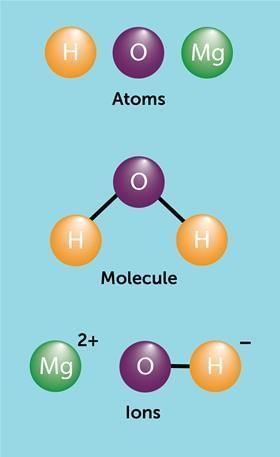
What defines a molecule and what is its smallest unit capable of independent existence? |
Card: 21 / 40 |
|
A molecule is the smallest particle of an element or a compound that can exist independently and shows all the properties of that substance. |
Card: 22 / 40 |
|
Atoms of different elements combine in definite proportions to form ___ of compounds. |
Card: 25 / 40 |
|
Valency determines how atoms of an element combine with atoms of another element to form a chemical compound. |
Card: 30 / 40 |
|
The chemical formula for Carbon Tetrachloride is CCl₄. It is derived from the valency of carbon, which is 4, and chlorine, which is 1, resulting in the formula CCl₄ through the crossover of their valencies. 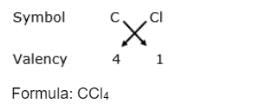 |
Card: 32 / 40 |
|
False. The correct formula for Magnesium Chloride is MgCl₂, derived from magnesium's valency of 2 and chlorine's valency of 1. |
Card: 34 / 40 |
|
Fill in the blank: The formula of Magnesium Chloride involves the crossover of the valencies of ___ and ___. |
Card: 35 / 40 |
|
What is the molecular mass of water (H₂O) calculated from its constituent atomic masses? |
Card: 37 / 40 |
|
The molecular mass of water is 18u, calculated as 2 × 1u (for hydrogen) + 16u (for oxygen). |
Card: 38 / 40 |
|
The formula unit mass of CaCl₂ is calculated as the sum of the atomic masses of which elements? |
Card: 39 / 40 |
|
The formula unit mass of CaCl₂ is calculated as the atomic mass of calcium (40u) plus twice the atomic mass of chlorine (2 × 35.5u), totaling 111u. |
Card: 40 / 40 |