|
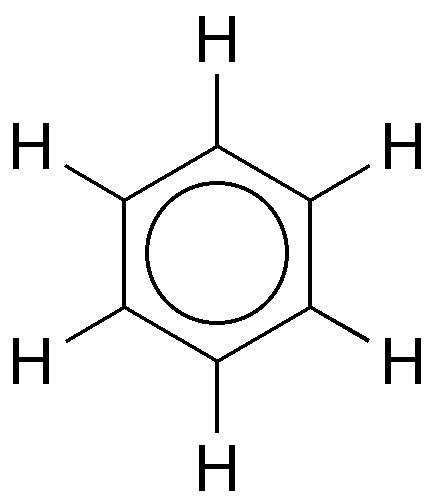
Aromatic hydrocarbons contain delocalized pi electrons in a cyclic structure, while aliphatic hydrocarbons do not have this resonance stabilization. |
Card: 2 / 38 |
|
Chlorophyll contains a porphyrin ring that exhibits ___ properties, essential for photosynthesis. |
Card: 3 / 38 |
|
True or False: Aromatic hydrocarbons generally have a hydrogen-to-carbon ratio of 1:1. |
Card: 5 / 38 |
|
Identify the aromatic hydrocarbon known for being a key ingredient in mothballs. |
Card: 9 / 38 |
|
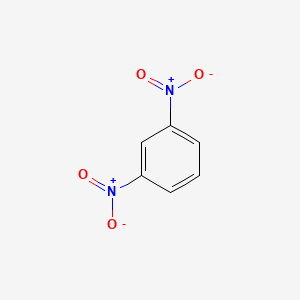
Fill in the blank: The first compound recognized as an aromatic hydrocarbon is ___. |
Card: 11 / 38 |
|
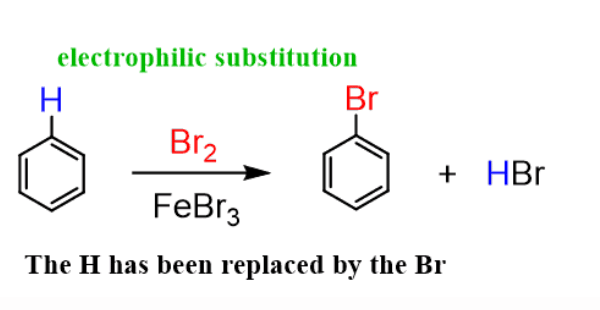
What is the primary type of reaction that aromatic hydrocarbons typically undergo? |
Card: 13 / 38 |
|
Riddle: I am a structure with rings that smell sweet or sour, I help in making plastics hour by hour. What am I? |
Card: 15 / 38 |
|
What is the role of Greek numerical prefixes like di, tri, and tetra in naming aromatic compounds? |
Card: 17 / 38 |
|
They indicate the number of identical substituent groups attached to the aromatic ring. |
Card: 18 / 38 |
|
What type of bond can be formed through coupling reactions involving aromatic hydrocarbons? |
Card: 19 / 38 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
Carbon-carbon bonds, carbon-oxygen bonds, and carbon-nitrogen bonds can be formed. |
Card: 20 / 38 |
|
Riddle: I am a hydrocarbon with a ring, I can be sweet or bitter, and for drugs, I often bring. What am I? |
Card: 23 / 38 |
|
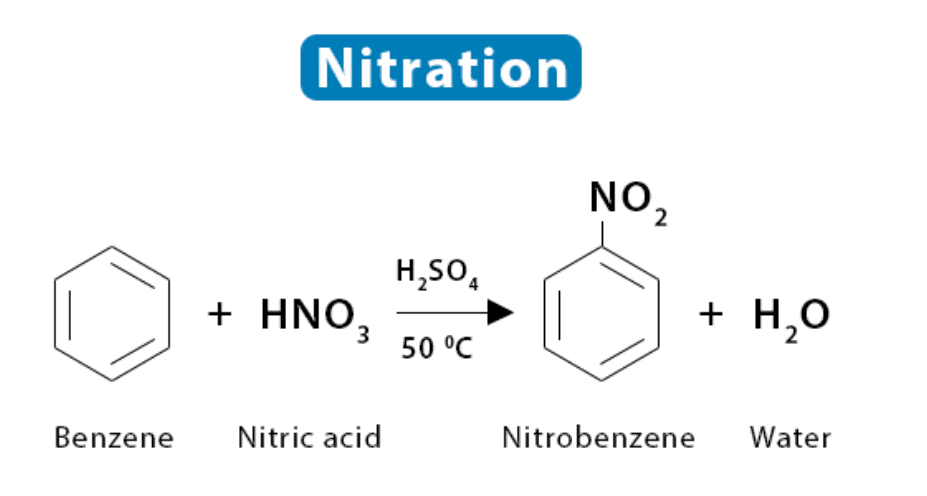
What is the common name for the reaction where a nitro group is introduced onto an aromatic ring? |
Card: 25 / 38 |
|
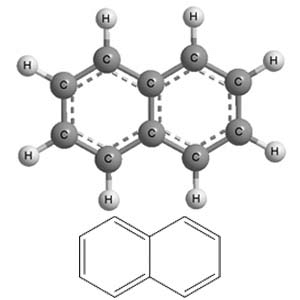
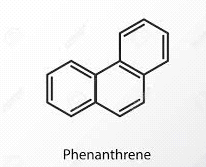
What type of aromatic hydrocarbon is defined as having multiple fused aromatic rings? |
Card: 27 / 38 |
|
Identify a common application of aromatic hydrocarbons in the petrochemical industry. |
Card: 31 / 38 |
|
What is the role of palladium(II) acetate in coupling reactions involving aromatic hydrocarbons? |
Card: 33 / 38 |
|
Fill in the blank: The hydrogen-to-carbon ratio characteristic of aromatic compounds is ___:___. |
Card: 35 / 38 |
|
How does the resonance in aromatic hydrocarbons contribute to their stability? |
Card: 37 / 38 |
|
Resonance allows for delocalization of pi electrons, which stabilizes the structure. |
Card: 38 / 38 |