|
Chemical kinetics is the branch of chemistry that studies the speed of chemical reactions and the factors that influence them. |
Card: 2 / 60 |
|
The reaction rate depends on concentration of reactants, temperature, presence of a catalyst, and surface area of reactants. |
Card: 4 / 60 |
|
Thermodynamics tells us whether a reaction is feasible, whereas kinetics tells us how fast it occurs |
Card: 6 / 60 |
|
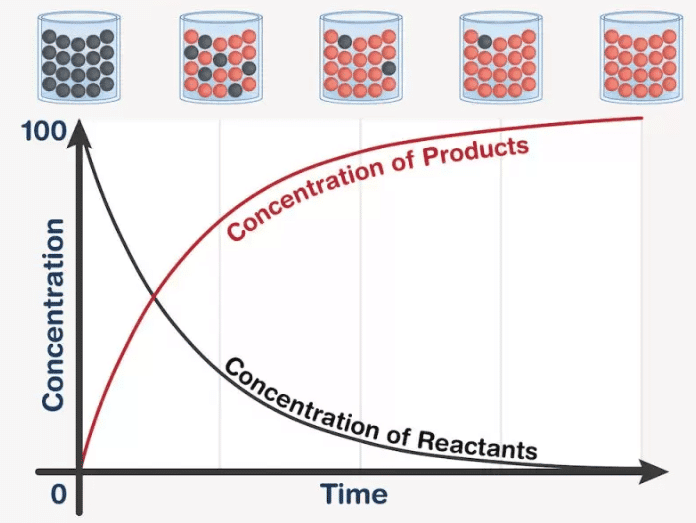
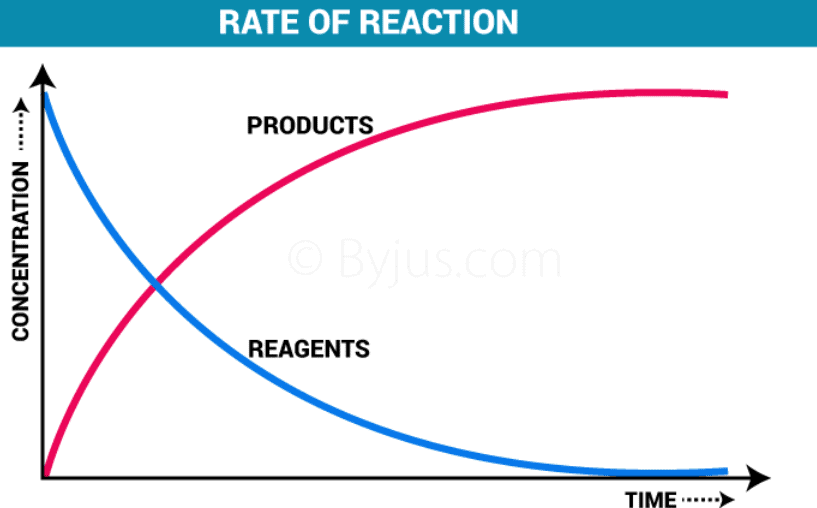
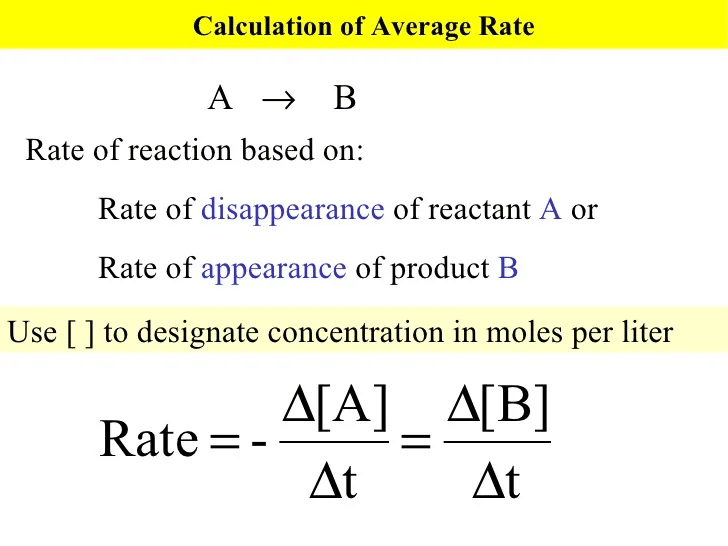
The rate of a reaction is the change in concentration of reactants or products per unit time. |
Card: 8 / 60 |
|
The units depend on concentration and time. For example, in terms of molarity (M): |
Card: 14 / 60 |
|
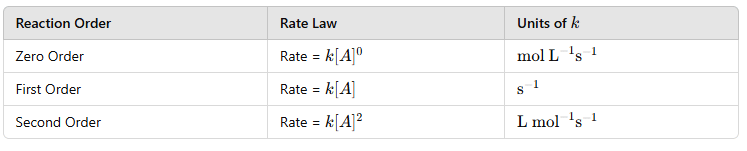
The order is determined experimentally and is not necessarily the same as the stoichiometric coefficients in the balanced equation. |
Card: 18 / 60 |
|
Card: 22 / 60 |
|
ln[A] = ln[A]0 − kt where [A] is the concentration at time t, [A]0 is the initial concentration, and k is the rate constant. |
Card: 26 / 60 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
k = Ae⁻ᴱᵃ/RT where k is the rate constant, A is the pre-exponential factor, Ea is the activation energy, R is the gas constant, and T is the temperature in Kelvin. |
Card: 34 / 60 |
|
ln k = -Ea/RT + ln A |
Card: 36 / 60 |
|
A catalyst lowers the activation energy by providing an alternate reaction pathway. |
Card: 38 / 60 |
|
Card: 40 / 60 |
|
The slowest step in a reaction mechanism, which controls the overall reaction rate. |
Card: 42 / 60 |
|
For a reaction to occur, molecules must:
|
Card: 44 / 60 |
|
The expression for the rate of reaction using collision theory is: |
Card: 46 / 60 |
|
It accounts for the probability of reactants colliding in the correct orientation. |
Card: 48 / 60 |
|
Card: 50 / 60 |
|
A reaction that appears to be first-order but actually follows a higher order because one reactant is in large excess. |
Card: 52 / 60 |
|
The hydrolysis of ethyl acetate in excess water: |
Card: 54 / 60 |
|
If a reaction follows second-order kinetics and the concentration of reactant is doubled, how does the rate change? |
Card: 57 / 60 |
|
It helps in predicting how long a reactant will take to reduce to half its original amount. |
Card: 60 / 60 |