|
Fill in the blank: The Periodic Table arranges elements in order of their increasing ___ number. |
Card: 3 / 30 |
|
True or False: Mendeleev's Periodic Law did not account for the position of isotopes. |
Card: 5 / 30 |
|
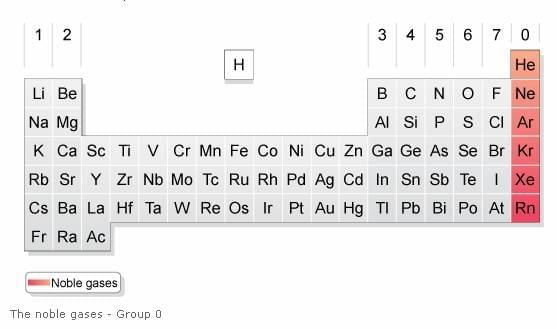
Anomalies included the position of hydrogen, rare earths, isotopes, noble gases, anomalous pairs of elements, and the cause of periodicity. |
Card: 8 / 30 |
|
Fill in the blank: The horizontal rows in the Periodic Table are referred to as ___ . |
Card: 9 / 30 |
|
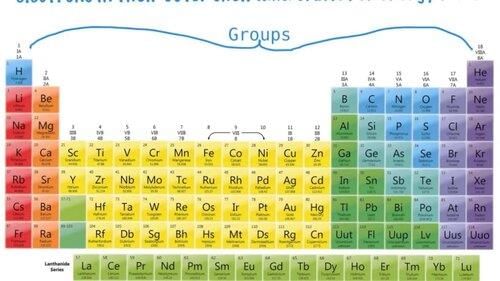
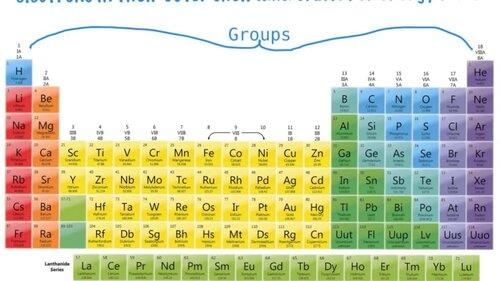
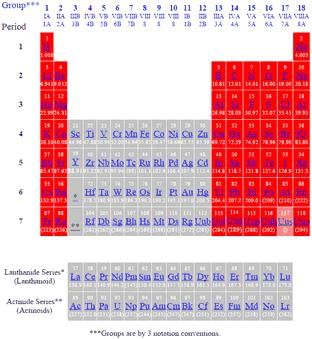
Vertical columns group elements.
|
Card: 12 / 30 |
|
A group is defined by the number of electrons present in the outermost shell of the elements.
|
Card: 14 / 30 |
 Unlock all Flashcards with EduRev Infinity Plan Starting from @ ₹99 only
|
|
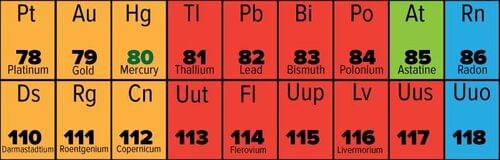
Fill in the blank: The sixth period of the periodic table contains ___ elements. |
Card: 19 / 30 |
|
The energy required to remove an electron from a neutral atom is known as ___ energy. |
Card: 25 / 30 |
|
Fill in the blank: The tendency of an atom to attract shared electrons in a molecule is referred to as its ___. |
Card: 27 / 30 |
|
Riddle: I am the measure of the distance from the nucleus to the outer shell, changing as elements vary. What am I? |
Card: 29 / 30 |