Free Radicals: Stability & Reactions | Organic Chemistry PDF Download
A. What is Free radicals?
(1) Carbon free radicals are odd electron species in which carbon atom bears the odd electron.
(2) Homolytic bond fission of a covalent single bond gives rise to free radicals.
(3) There are seven electrons in the outermost orbit of carbon of carbon free radicals.
(4) Owing to the presence of an odd electron; a carbon radical is paramagnetic in nature. Due to this reason free radicals are highly reactive.
(5) The structure of the carbon free radicals are very difficult to predict. They have planar to pyramidal shape depending upon the groups and atoms attached to the carbon atom having odd radicals is pyramidal. Alkyl free radicals have also pyramidal shape.
(6) Free radicals are neutral electrophiles.
(7) Free radicals generally reacts with free radical or neutral molecule.
B. Generation of Free radicals
Chemical reaction which takes place inthe presence of HELPER [H: Heat (≥5000C), E: Electricity, L : Light (sun light, hv), P: Peroxide, R: Radical], is known as free radical reaction.
Characteristics of free radical reactions
(i) Reaction intermediate is free radical
(ii) Product formation takes place by formation of most stable free radical.
C. Fate of free radicals:
Radical reaction takes place in three steps:
(a) Initiation (b) Propagation (c) Termination.
(i) Initiation step:
In this step, homolytic bond fission takes place in the presence of initiator, i.e., peroxide, hv,heat etc. The process is always endothermic.
(ii) Propagation step: Propagation step is always two or more than two step process and all propagation steps should be exothermic, otherwise free radical reaction would not take place.
Propagation steps I: In this step, formation of free radical as reaction intermediate takes place.
For exaple,
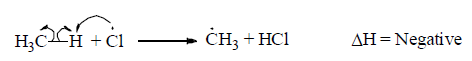
Propagation step II: Reaction intermediate reacts with reagent to give the product.
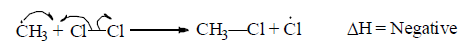
(iii) Termination step: In this step, free radical formed in the last propagation step (generally propagation step II) is destroyed by the addition or by the addition of some impurities like CHCl3 or CCl4.
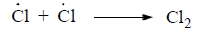
(iv) Certaion compounds, known as inhibitor, retared velocity of free radical reactions. Common inhibitors are O2, I2, p-Benzoquinone and diphynylamine.
(v) Amount of energy needed for homolysis of a covalent bond depends upon the stability of resulting free radical as reaction intermediate (RI)
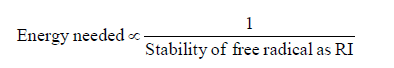
D. Stability of Free radical
(a) Stability of Alkyl free radicals: Stability of alkyl free radicals can be explained by hyperconjugation and number of resonating structures due to the hyperconjugation. The decreasing order of stability of alkyl free radicals is as follows:
tertiary free radical > secondary free radical > primary free radical > CH3.
Note: Both electron withdrawing groups such as carbonyl, cyano and nitro and electro-donating groups such as methoxy and dimethyl amino have a stabilising effect on a radical at an adjacent carbon due to resonance.
(b) Stability of Allyl and Benzyl free radicals:
(i) Stability of these radicals can be explained by delocalisation of electrons or resonance.
Structure: (C6H5)3C > (C6H5)2CH > C6H5 CH2 > CH2=CH—CH2
No. of resonating structures:
(ii) Allyl and bezyl radicals are more stable than alkyl radicals. Triphenylmethyl radical and similar radicals are stable enough to exist in solution at room temperature.
(c) Stability of different free radicals in decreasing order:
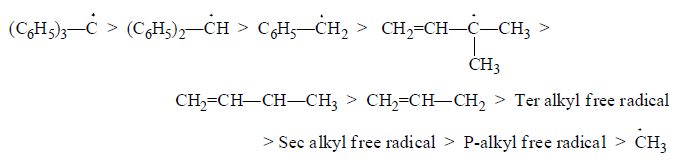
(d) Stability of the same type of alkyl free radical ∝ Number of carbons in alkyl free radical.
(e) Rearrangement takes place in primary-free radical in a chemical reaction.
4. BENZYNE
A. What is Benzyne
(i) 1, 2-Didehydrobenzene, C6H4, and its derivatives are called benzyne or arynes and the simplest member is benzyne.
(ii) It is neutral reaction intermediate derived from benzene ring by removing two substituents, of ortho positions, one in the form of electrophile and other in the form of nucleophile leaving behind two electrons to be distributed between two orbitals.
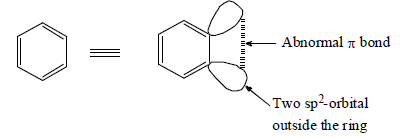
(iii) Benzyne intermediate is aromatic in character.
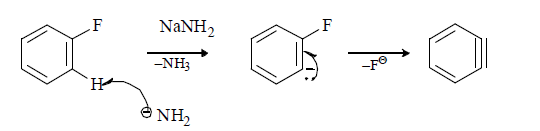
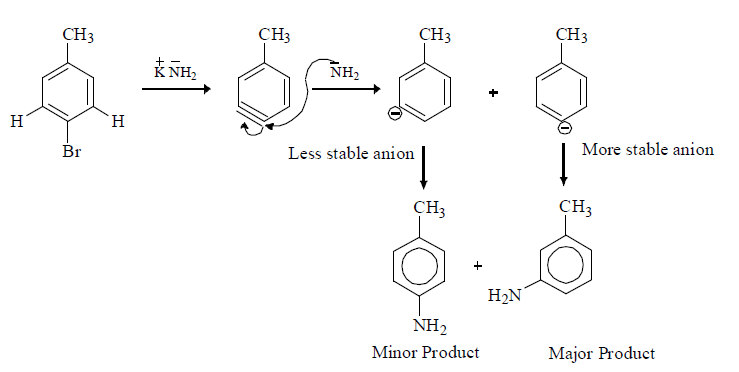
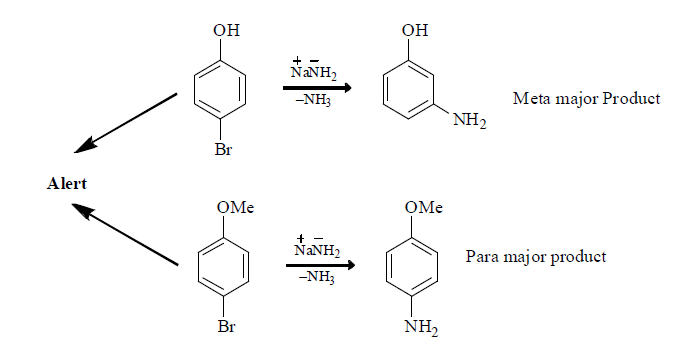
(iv) When halobenzene is heated with sodamide formation of benzyne takes place.
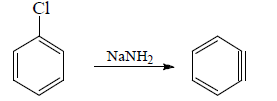
(i) It behaves as dienophile and gives Diels-Alder reaction with diene.
(ii) It reacts with strong nucleiophile like NH2.
Structure of Benzyne:(1) The triple bond of benzyne is actually not a pure triplet bond because sp hydrogen with 180° is not possible for ring having less than 8 carbon therefore the C atom of benzyne are sp2 hybridized. Due to high reactivity of benzyne intermediate, cannot be isolated. However benzyne can be trapped in to other forms by Diels Alder reaction.
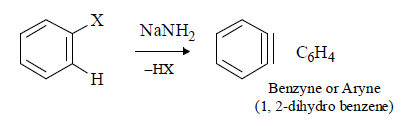
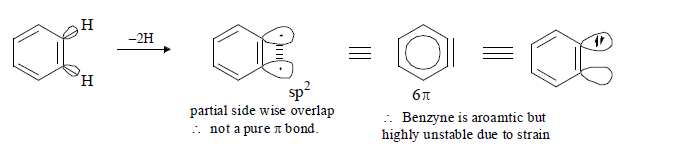
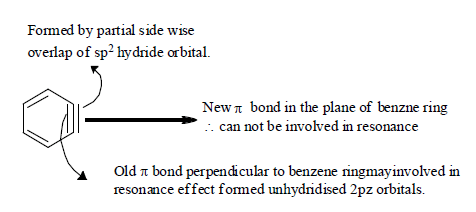
B) Generation of Benzyne
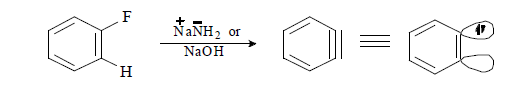
Mechanism of Benzyne formation:
(2) From anthranilic acid (o-amino benzoic acid)
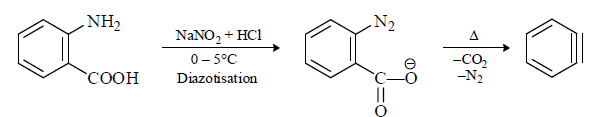
(3) From special compounds: Having CO2, CO, SO2 etc.
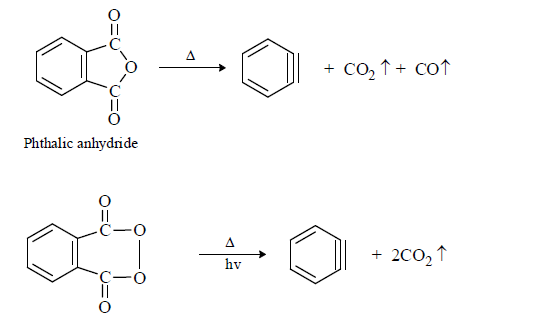
The driving force of these reaction is release of CO, CO2, SO3 gas (SO3 is stable than SO2)
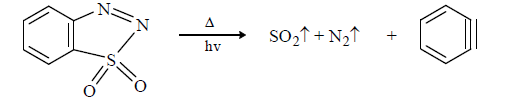
(4) From halo benzene with Mg/Li
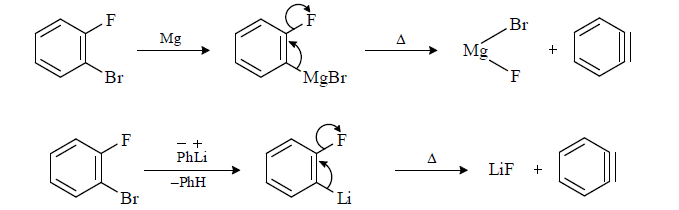
Li due to its small size show polarization effect and form bond polar covalent bond with ring while K does not form polar covalent bond.
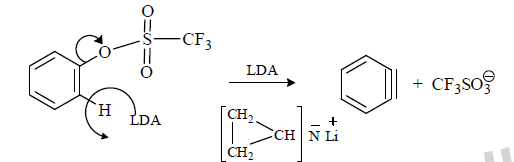
(5) Modern method: The most recent method for the preparation of benzyne is LDA and Triflath.
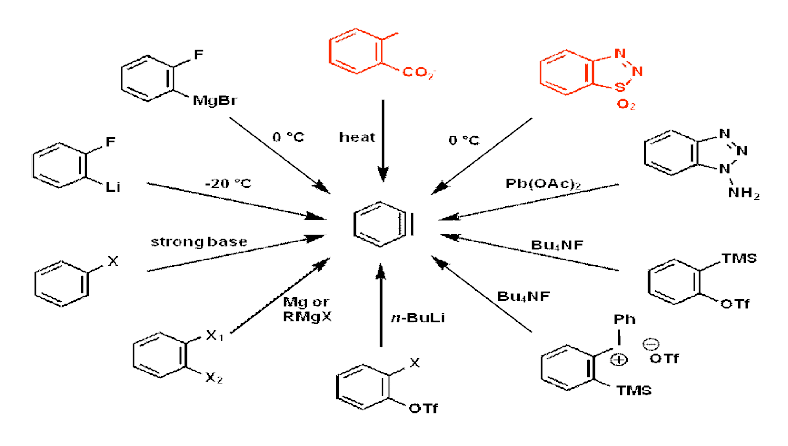
Summary of Methods of Preparation of Benzyne:
(C) Fate of Benzyne: Benzyne can react as both nucleophile and Electrophile
(1) Addition Elimination Reaction:
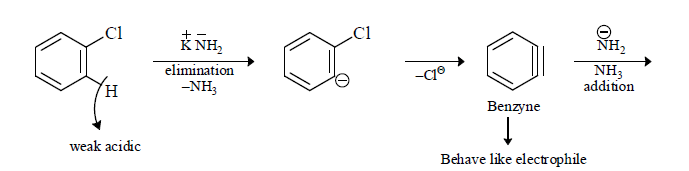
∴ To release this very strong base is required like 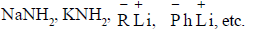
Example
Reaction with Grignard:
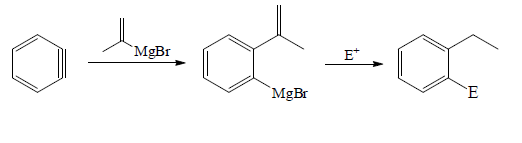
Reaction with Organolithium reagents:
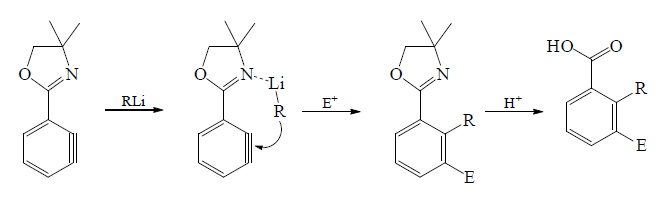
Reaction of Copper-lithium reagents:
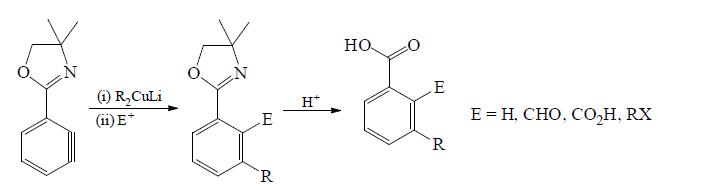
Trapping of Benzyne intermediate: By Diels Alder reaction and dimerisation reaction.In D.A. reaction benzyne behaves as dienophile.
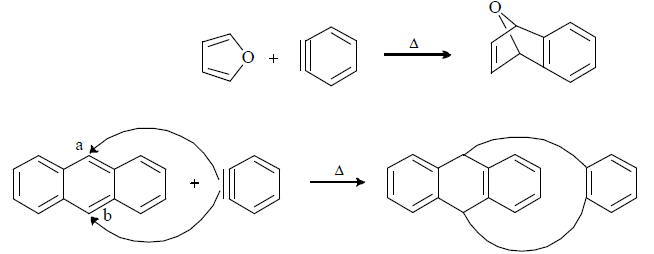
|
44 videos|102 docs|52 tests
|
FAQs on Free Radicals: Stability & Reactions - Organic Chemistry
| 1. What are free radicals and why are they important in chemistry? |  |
| 2. How do free radicals attain stability? |  |
| 3. What are the common reactions involving free radicals? |  |
| 4. How do free radicals contribute to aging and diseases? |  |
| 5. How can we control free radicals in our daily lives? |  |





















