General concepts of Group Relationships and Gradation in Properties | Inorganic Chemistry PDF Download
S and P Block Elements
Chemistry is the basically related with the study of elements.These elements are classified in some categories and the two most important categories of the elements are known as representative elements.
Representative elements of the periodic table include s are p block elements.
s-block elements included the elements of group 1 and group 2 of the periodic table while
p – block elements include the elements of group 13, 14, 15, 16, 17 and 18.
s- block elements arte all metals on the other hand p- block elements are mainly non-metals but they include some metalloids and metals also.
The s and b lock elements have a great role in chemistry and also our life. As we will study the properties of all these one by one in this chapter will be able to know about the great importance and applications of these elements.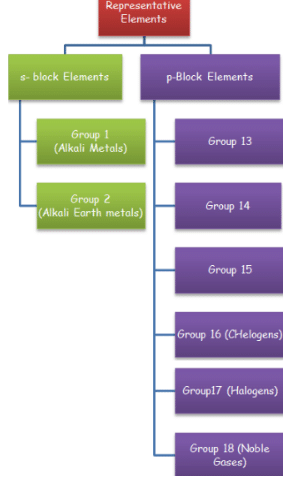
- Alkali Metals
- Lithium
- Sodium
- Compounds of Alkali Metals
- Alkaline Earth Metals
- Compounds of Alkali Earth Metals
- Magnesium
- Beryllium
- Solved Problems
The elements in the long form of the periodic table has been divided into four blocks, namely s, p, d & f blocks. The elements of group I & II receive their last electron in s-orbital. So they are called as s – block elements.
The metals Lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs) and francium (Fr) which have one electron in their outermost shell belongs to group I and are called alkali metals as they react with water to form hydroxides which are strong bases or alkalies.
The elements of group II are Berryllium (Be), Magnesium (Mg), Calcium (Ca), Strontium (Sr), barium (Ba) and radium (Ra) which have two electrons in their outermost shell.
All these elements are also metallic in nature and are commonly known as alkaline earth metals with the exception of beryllium. Because of their low density, alkali metals and alkaline earth metals are called lighter metals.
Both alkali and alkaline earth metals are highly reactive and hence do not occur in free state but found in combined state. Whereas alkali metals mostly occur as halides, oxides, silicates, borates and nitrates, alkaline earth metals mainly occur as silicates, carbonates, sulphates and phosphates.
Some alkali & alkaline earth metals occur abundantly in nature. Calcium is the fifth, magnesium is the sixth, sodium is seventh and potassium is eight barium is the fourteenth and strontium is the fifteenth most abundant element by weight in the earth’s crust. Sodium and magnesium are also present in sea water brine wells and few salt lakes.
Anamolous behavour of first element
The first element of a group differs considerably from the rest of the elements of the same group. This anomolous behaviour is due to
- Smaller size of their atoms
- Their higher ionization energies
- Their higher electro-negativites
- Absence of vacant d – orbitals in their valence shell
- High polarizing power of its cation.
Thus Li differes from the rest of alkali metals (Na, K, Rb & Cs) and Be differs from rest of the alkaline earth metals (Mg, Ca, Sr & Ba)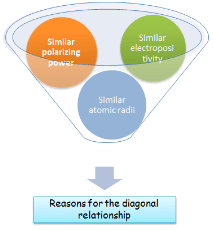
Diagonal Relationships
On moving diagonally some members show similar properties with the members of next higher group which is particularly seen in the elements of second and third periods of the periodic table. However the similarities shown are far less pronounced than the similarities with in a group.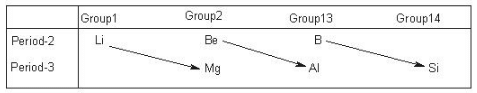
The main reasons for the diagonal relationship are
- Similarity in electropositive character: The electropositive character decreases along a period, but increases down a group. Hence on moving diagonally the two opposing trend partially cancels out. As a result diagonally related elements have similar electropositive character and hence exhibit similar properties.
- Similarity in polarizing power: On moving along a period from left to right, the charge on the ions increases while ionic size decreases, hence polarizing power increases. On moving down the group, the ionic size increases and hence polarizing power decreases. On moving diagonally, these two trends partially cancel out. As a result thus diagonally related elements have same polarizing power and thus exhibit similar properties.
- Similarity in atomic or ionic radii: The atomic and ionic radii decrease across a period and increase in a group. Evidently on moving diagonally, the two trends partially cancel out. As a result, diagonally related elements have similar atomic & ionic radii and hence have similar properties.
|
50 videos|92 docs|41 tests
|
FAQs on General concepts of Group Relationships and Gradation in Properties - Inorganic Chemistry
| 1. What are s and p block elements? |  |
| 2. What are the general concepts of group relationships in s and p block elements? |  |
| 3. How do the properties of s and p block elements vary across a period? |  |
| 4. What is gradation in properties in s and p block elements? |  |
| 5. How do the properties of s and p block elements affect their chemical reactivity? |  |
















