Chemistry Exam > Chemistry Notes > Organic Chemistry > Group Transfer & Metallo-Ene Reactions
Group Transfer & Metallo-Ene Reactions | Organic Chemistry PDF Download
GROUP TRANSFER REACTIONS
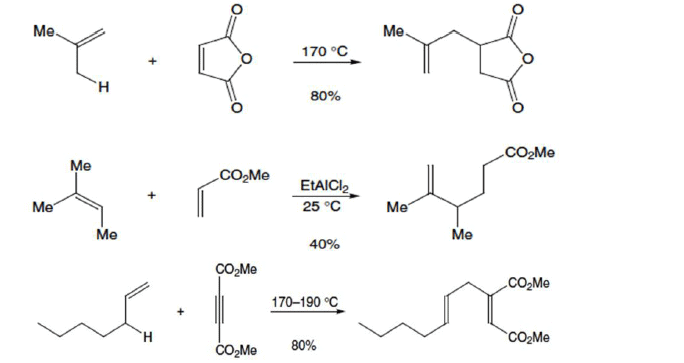
It involves the activation of an allylic C—H bond and the allylic transposition of the C = C bond of readily available alkenes. This reaction is known as the ene reaction. Formally, it is the addition of alkenes to double bonds (C = C or C = O), and it is one of the simplest ways to form C-C bonds.
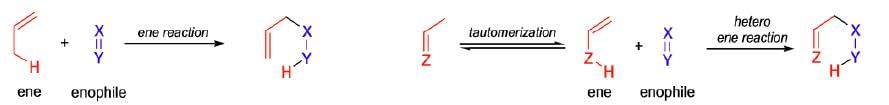
Mechanism:
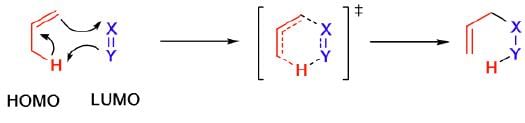
Example
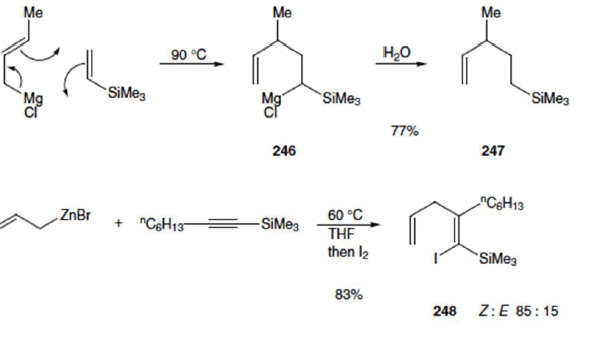
Metallo-Ene Reactions
Allylic metal reagents (e.g. metals Mg, Zn, Li, Ni, Pd, Pt) take part readily by migration of the metal atom (instead of a hydrogen atom) and formation of a new carbon–metal bond.
The document Group Transfer & Metallo-Ene Reactions | Organic Chemistry is a part of the Chemistry Course Organic Chemistry.
All you need of Chemistry at this link: Chemistry
|
44 videos|102 docs|52 tests
|
FAQs on Group Transfer & Metallo-Ene Reactions - Organic Chemistry
| 1. What is group transfer in organic chemistry? |  |
Group transfer in organic chemistry refers to a reaction in which a functional group is transferred from one molecule to another. It involves the breaking and formation of chemical bonds. This process is commonly used in organic synthesis to introduce or transform functional groups in a molecule.
| 2. How do metallo-ene reactions occur? |  |
Metallo-ene reactions occur when a metal catalyst is involved in the addition of an alkene to a metal-carbon bond. The alkene adds to the metal-carbon bond, resulting in the formation of a new carbon-carbon bond. This reaction is an important tool in organic synthesis as it allows for the formation of complex molecules with high stereo- and regioselectivity.
| 3. What are the applications of group transfer reactions? |  |
Group transfer reactions have various applications in organic synthesis. They can be used to introduce specific functional groups into a molecule, modify existing functional groups, or create new carbon-carbon bonds. These reactions are widely employed in the pharmaceutical, agrochemical, and materials industries for the production of complex organic compounds.
| 4. Can metallo-ene reactions be used for asymmetric synthesis? |  |
Yes, metallo-ene reactions can be utilized for asymmetric synthesis. By using chiral ligands or catalysts, it is possible to control the stereochemistry of the newly formed carbon-carbon bond. This allows for the selective formation of enantiopure products, which is crucial in the synthesis of pharmaceuticals and other bioactive compounds.
| 5. What are the advantages of using metallo-ene reactions in organic synthesis? |  |
Metallo-ene reactions offer several advantages in organic synthesis. They provide high regio- and stereocontrol, allowing for the selective formation of specific carbon-carbon bonds. These reactions are compatible with a wide range of functional groups and can be conducted under mild reaction conditions. Furthermore, metal catalysts can be easily recovered and reused, making the process more environmentally friendly.
Related Searches

















