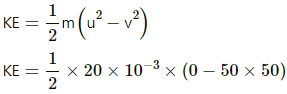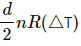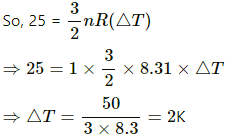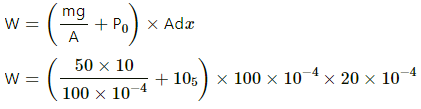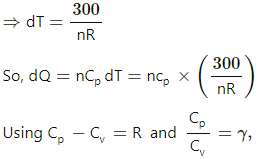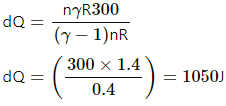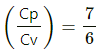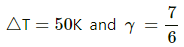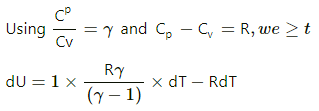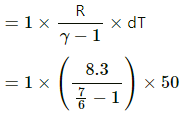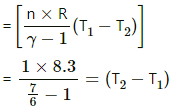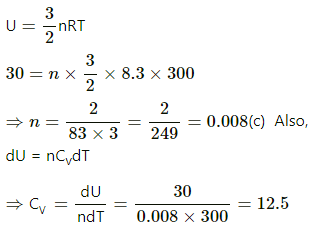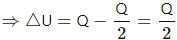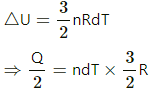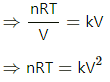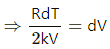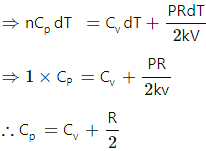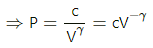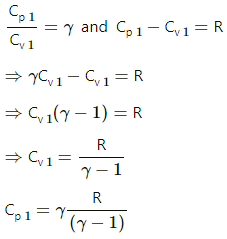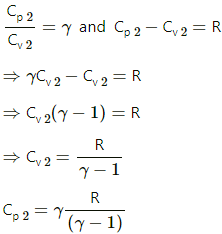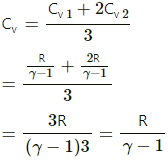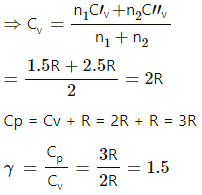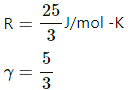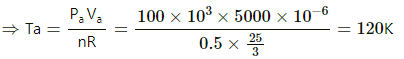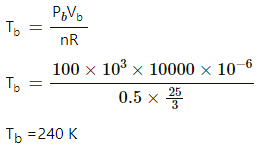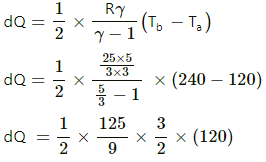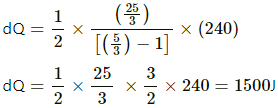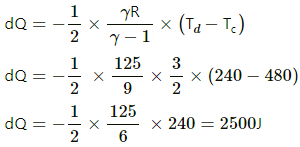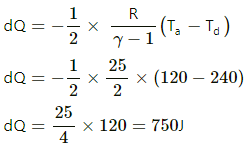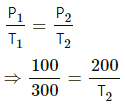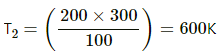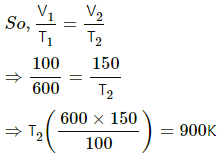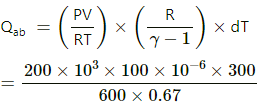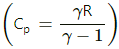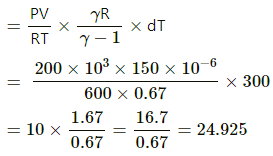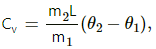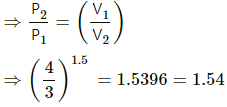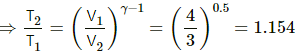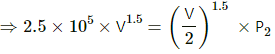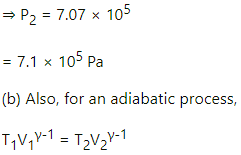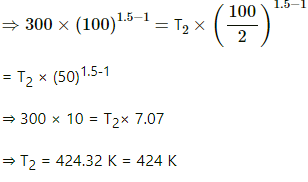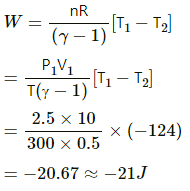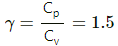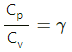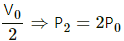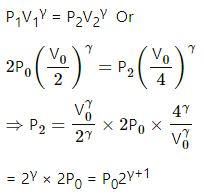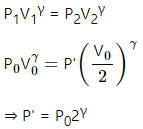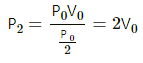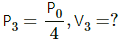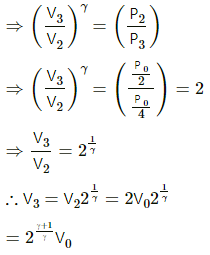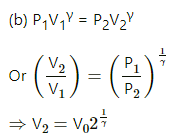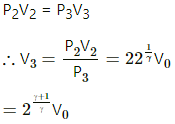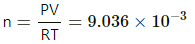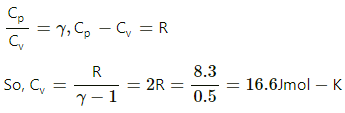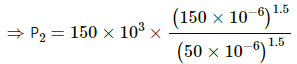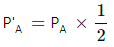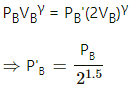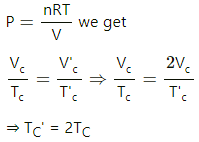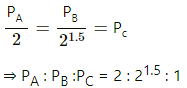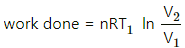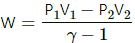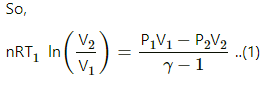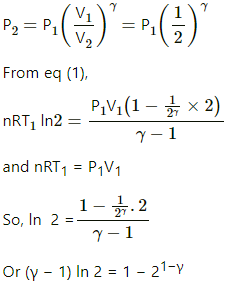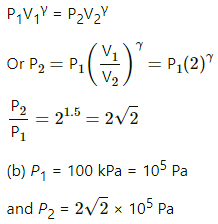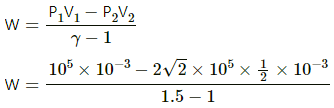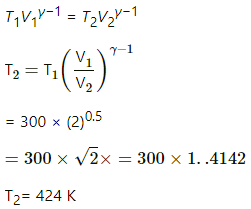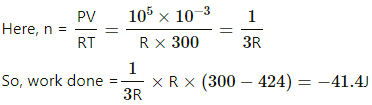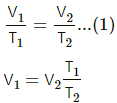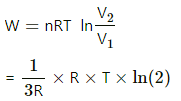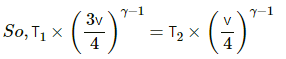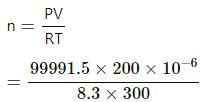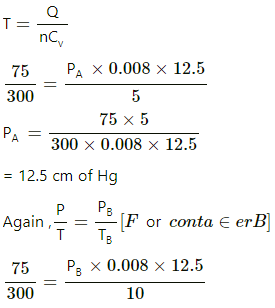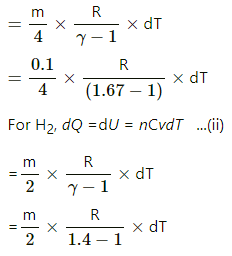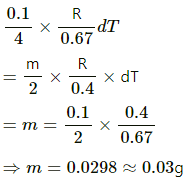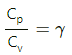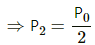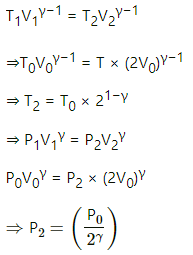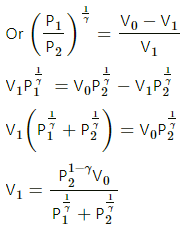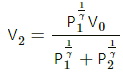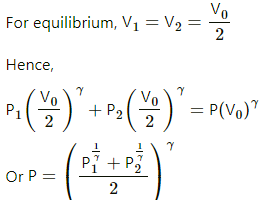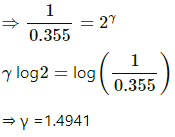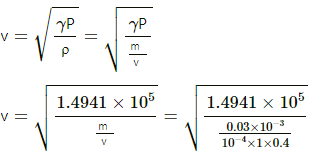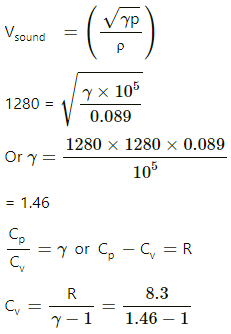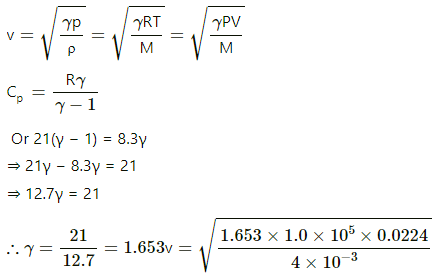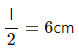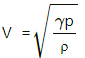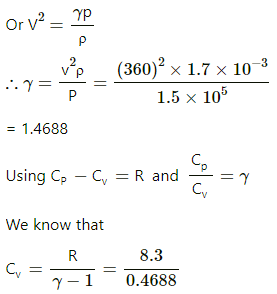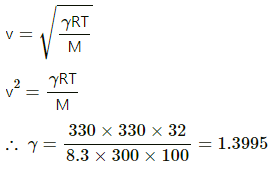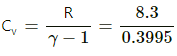HC Verma Questions and Solutions: Chapter 27: Specific Heat Capacities of Gases- 2 | HC Verma Solutions - JEE PDF Download
Exercises
Q.1. A vessel containing one mole of a monatomic ideal gas (molecular weight = 20 g mol−1) is moving on a floor at a speed of 50 m s−1. The vessel is stopped suddenly. Assuming that the mechanical energy lost has gone into the internal energy of the gas, find the rise in its temperature.
Number of moles of the ideal gas, n = 1 mole
Molecular weight of the gas, W = 20 g/mole
Mass of the gas, m =20 g
Velocity of the vessel, V = 50 m/s
Decrease in K.E. of the vessel = Internal energy gained by the gas
KE = -25 J = gain in internal energy of the gas change in internal energy of a gas
where d is the degree of freedom of the gas F or a monoatomic gas , d=3.
Q.2. 5 g of a gas is contained in a rigid container and is heated from 15°C to 25°C. Specific heat capacity of the gas at constant volume is 0.172 cal g−1 °C−1 and the mechanical equivalent of heat is 4.2 J cal−1. Calculate the change in the internal energy of the gas.
Given:
Mass of the gas, m = 5 g
Change in temperature of the system, ∆T = 25 − 15°C = 10°C
Specific heat at constant volume, Cv = 0.172 cal/g -°C
Mechanical equivalent, J = 4.2 J/cal
From the first law of thermodynamics,
dQ = dU +dW
Now ,
ΔV = 0 (Rigid wall of the container keeps the volume constant)
So, dW =PΔV =0
Therefore,
dQ =dU (From the first law)
Q = mcvdT = 5×0.172×10
= 8.6 cal = 8.6 × 4.2 J
= 36.12 J
So, change in internal energy of the system is 36.12 J.
Q.3. The figure shows a cylindrical container containing oxygen (γ = 1.4) and closed by a 50-kg frictionless piston. The area of cross-section is 100 cm2, atmospheric pressure is 100 kPa and g is 10 m s−2. The cylinder is slowly heated for some time. Find the amount of heat supplied to the gas if the piston moves out through a distance of 20 cm.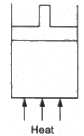
Given:
Mass of the piston (m) = 50 kg
Adiabatic constant of the gas, γ = 1.4
Area of cross-section of the piston (A) = 100 cm2
Atmospheric pressure (P0) = 100 kPa
g = 10 m/s2
Distance moved by the piston , x = 20 cm
Work done by the gas,
dW=Pdv
The pressure (p) is because of two factors : the first is the initial pressure and
Therefore,
W =( 5 × 104 +105) × 20 × 10-4
W = 1.5 × 105 × 20 × 10-4
W = 300 J
Hence , nRdT = PΔV = 300
Q.4. The specific heat capacities of hydrogen at constant volume and at constant pressure are 2.4 cal g−1 °C−1 and 3.4 cal g−1 °C−1 respectively. The molecular weight of hydrogen is 2 g mol−1 and the gas constant, R = 8.3 × 107 erg °C−1 mol−1. Calculate the value of J.
Specific heat capacity at constant volume, Cv(H2) = 2.4 cal/g-°C
Specific heat capacity at constant pressure, Cp(H2) = 3.4 cal/g-°C
Molecular weight, M = 2 g/mol
Gas constant, R = 8.3 × 107 erg/mol-°C
We know: Cp − Cv = 1 cal/g-°C,
where Cp and Cv are molar specific heat capacities.
So, difference of molar specific heat,
Cp × M − Cv × M = 1 cal/mol-°C
Now, 2 × J = R
⇒ 2 × J = 8.3 × 107 erg/mol-°C
⇒ J = 4.15 × 107 erg/cal
Q.5. The ratio of the molar heat capacities of an ideal gas is Cp/Cv = 7/6. Calculate the change in internal energy of 1.0 mole of the gas when its temperature is raised by 50 K (a) keeping the pressure constant (b) keeping the volume constant and (c) adiaba.
Given:
Number of moles of the gas, n = 1 mol
Change in temperature of the gas, ∆T = 50 K
(a) Keeping the pressure constant:
Using the first law of thermodynamics,
dQ= dU+dW
dQ =dU +dW
work done , dW =PdV
As pressure is kept constant, work done = P(Δ V)
Using the ideal gas equation PV =nRT,
P(ΔV) =nR(ΔT)
⇒ dW =nR(ΔT)
At constant pressure, dQ=nCpdT
Substituting these values in the first law of thermodynamics, we get
nCp dT = dU + RdT
⇒ dU = nCp dT − RdT
= 7 RdT − RdT
= 7 RdT − RdT = 6 RdT
= 6 × 8.3 × 50 = 2490 J
(b) Keeping volume constant:
Work done = 0
Using the first law of thermodynamics,
dU = dQ
dU = nCv dT
= 8.3 × 50 × 6 = 2490 J
(c) Adiabatically, dQ = 0
Using the first law of thermodynamics, we get
dU = − dW
= 8.3 × 6 × 50 =2490 J
Q.6. A sample of air weighing 1.18 g occupies 1.0 × 103 cm3 when kept at 300 K and 1.0 × 105 Pa. When 2.0 cal of heat is added to it at constant volume, its temperature increases by 1°C. Calculate the amount of heat needed to increase the temperature of air by 1°C at constant pressure if the mechanical equivalent of heat is 4.2 × 107 erg cal−1. Assume that air behaves as an ideal gas.
Here,
m = 1.18 g = 1.18×10-3 kg
ΔQ = 2.0×4.2 J
P = 1.0×106 Pa
V = 1.0×103 cm3 = 1.0×10-3 m3
T = 300K
Applying eqn. of state
PV = nRT
⇒ n = PV/RT
⇒ n = 1.0×105×1.0×10-3/(8.314×300)
⇒ n = 0.04
ΔT = 1°C
(ΔH)v = nCv ΔT
2.0×4.2 = nCv×1
⇒ Cv = 8.4/n= 8.4/0.04
⇒ Cv = 210
Again we know
Cp – Cv =R
⇒ Cp = R + Cv
⇒ Cp = 8.3 + 210
⇒ Cp = 218.3
Now at constant pressure
(ΔH)p = nCp ΔT
⇒ (ΔH)p = 218.3×0.04×1 = 8.732 J
In calories
⇒ (ΔH)p = 8.732/4.2 = 2.08 cal
Q.7. An ideal gas expands from 100 cm3 to 200 cm3 at a constant pressure of 2.0 × 105 Pa when 50 J of heat is supplied to it. Calculate (a) the change in internal energy of the gas (b) the number of moles in the gas if the initial temperature is 300 K (c) the molar heat capacity Cp at constant pressure and (d) the molar heat capacity Cv at constant volume.
Initial volume of the gas, V1 = 100 cm3
Final volume = V2 = 200 cm3
Pressure = 2 × 105 Pa
Heat supplied, dQ = 50 J
(a) According to the first law of thermodynamics,
dQ = dU + dW
dW = PΔV = 2 × 105 × (200 -100) × 10-6 = 20
Initial volume of the gas, V1 = 100 cm3
Final volume = V2 = 200 cm3
Pressure = 2 × 105 Pa
Heat supplied, dQ = 50 J
(a) According to the first law of thermodynamics,
dQ = dU + dW
Cp = Cv + R = 12.5 + 8.3 = 20.8 J/mol-K
(d) Cv = 12.5 J/mol-K
Q.8. An amount Q of heat is added to a monatomic ideal gas in a process in which the gas performs a work Q/2 on its surrounding. Find the molar heat capacity for the process.
Given:
Amount of heat given to the gas = Q
So, ∆Q = Q
Work done by the gas, Δ W = Q/2
From the first law of thermodynamics,
ΔQ = ΔW +Δ U
For a monoatomic gas,
⇒ Q = 3nRdT
Again, for expansion at constant pressure,
Q = nCpdT,
where Cp is the molar heat capacity at constant pressure.
So, 3RndT = nCpdT
⇒ Cp = 3R
Q.9. An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation p = kV. Show that the molar heat capacity of the gas for the process is given by C = Cv + (R/2).
Relation between pressure and volume of a gas is P = kV.
Ideal gas equation is PV = nRT.
For simplicity, take the number of moles of a gas, n = 1.
⇒ RdT = 2 kVdV
From the first law of thermodynamics,
dQ = dU + dW
⇒ nCPdT = CVdT + PdV
Q.10. An ideal gas (Cp / Cv = γ) is taken through a process in which the pressure and the volume vary as p = aVb. Find the value of b for which the specific heat capacity in the process is zero.
As the process has specific heat capacity zero, the process is essentially an adiabatic process.
For an adiabatic process
PVγ = c
Comparing with eqn.
P = aVb
⇒ a = c
⇒ b = -γ
Q.11. Two ideal gases have the same value of Cp / Cv = γ. What will be the value of this ratio for a mixture of the two gases in the ratio 1 : 2?
For the first ideal gas,
Cp1 = specific heat at constant pressure
Cv1 = specific heat at constant volume
n1 = number of moles of the gas
For the second ideal gas,
Cp2 = specific heat at constant pressure
Cv2 = specific heat at constant volume
n2 = number of moles of the gas
Given:
n1 = n2 = 1 : 2
dU1 = nCv1dt
dU2= 2nCv2dT
When the gases are mixed,
nCv1dT + 2nCv2dT = 3nCvdT
Hence, Cp/Cv in the mixture is γ.
Q.12. A mixture contains 1 mole of helium (Cp = 2.5 R, Cv = 1.5 R) and 1 mole of hydrogen (Cp= 3.5 R, Cv = 2.5 R). Calculate the values of Cp, Cv and γ for the mixture.
Specific heat at constant pressure of helium, Cp' = 2.5 R
Specific heat at constant pressure of hydrogen, Cp" = 3.5 R
Specific heat at constant volume of helium, Cv' = 1.5 R
Specific heat at constant volume of hydrogen, Cv" = 2.5 R
n1 = n2 = 1 mol
dU = nCvdT
For the mixture of two gases,
dU1 +dU2 = 1 mol
[n1 + n2] CvdT = n1C'vdT + n2C"vdT,
where Cv is the heat capacity of the mixture
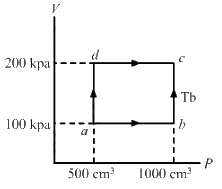
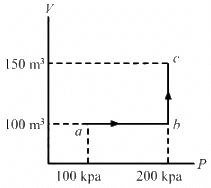
Q.13. Half mole of an ideal gas (γ = 5/3) is taken through the cycle abcda, as shown in the figure. Take R = 25/3 JK-1mol-1. (a) Find the temperature of the gas in the states a, b, c and d. (b) Find the amount of heat supplied in the processes ab and bc. (c) Find the amount of heat liberated in the processes cd and da.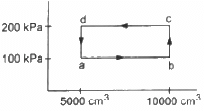
Given:
Number of moles of the gas,
n = 0.5 mol
(a) Temperature at a = Ta
PaVa =nRTa
Similarly, temperature at b,
Similarly, temperature at c is 480 K and at d is 240 K.
(b) For process ab,
dQ = ncpdT
[Since ab is isobaric]
dQ = 1250 J
For line bc, volume is constant. So, it is an isochoric process.
dQ = dU + dW
[dW = 0, isochoric process]
dQ = dU = nCvdT
dQ = nCv (Tc - Tb)
(c) Heat liberated in cd (isobaric process),
dQ = − nCpdT
Heat liberated in da (isochoric process),
⇒ dQ = dU
Q= −nCvdT
Q.14. An ideal gas (γ = 1.67) is taken through the process abc shown in the figure. The temperature at point a is 300 K. Calculate (a) the temperatures at b and c (b) the work done in the process (c) the amount of heat supplied in the path ab and in the path bcand (d) the change in the internal energy of the gas in the process.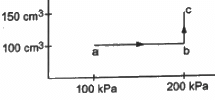
(a) For line ab, volume is constant.
So, from the ideal gas equation,
For line bc, pressure is constant.
(b) As process ab is isochoric, Wab =0 .
During process bc,
P = 200 kPa
The volume is changing from 100 to 150 cm3 .
Therefore, work done = 50 × 10−6 × 200 × 103 J= 10 J
(c) For ab (isochoric process), work done = 0.
From the first law,
dQ = dU = nCvdT
⇒ Heat supplied = nCvdT
Now,
= 14.925 ( ∴ γ = 1.67)
F or bc (isobaric process) :
Heat supplied in bc = nCpdT
(d) dQ = dU + dW
Now,
dU = dQ − dW
= Heat supplied − Work done
= (24.925 + 14.925) − 10
= 39.850 − 10 = 29.850 J.
Q.15. In Joly's differential steam calorimeter, 3 g of an ideal gas is contained in a rigid closed sphere at 20°C. The sphere is heated by steam at 100°C and it is found that an extra 0.095 g of steam has condensed into water as the temperature of the gas becomes constant. Calculate the specific heat capacity of the gas in J g−1 K−1. The latent heat of vaporisation of water = 540 cal g−1 .
For Joly's differential steam calorimeter,
where
m2 = mass of steam condensed
m2 = 0.095 g
Latent heat of vapourization, L = 540 cal/g = 540 × 4.2 J/g
m1 = mass of gas present
m1 = 3 g
Initial temperature, θ1 = 20°C
Final temperature, θ2 = 100°C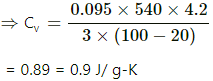
Q.16. The volume of an ideal gas (γ = 1.5) is changed adiabatically from 4.00 litres to 3.00 litres. Find the ratio of (a) the final pressure to the initial pressure and (b) the final temperature to the initial temperature.
Given,
γ = 1.5
Since the process is adiabatic, PVγ = constant.
(a) P1V1γ = P2V2γ
Given, V1 = 4 L
V2 = 3 L
we need to find P2/P1.
(b) Also, for an adiabatic process,
TVγ−1 = constant
T1V1γ−1 = T2V2γ−1
Q.17. An ideal gas at pressure 2.5 × 105 Pa and temperature 300 K occupies 100 cc. It is adiabatically compressed to half its original volume. Calculate (a) the final pressure (b) the final temperature and (c) the work done by the gas in the process. Take γ = 1.5.
Initial pressure of the gas, P1 = 2.5 × 105 Pa
Initial temperature, T1 = 300 K
Initial volume, V1 = 100 cc
(a) For an adiabatic process,
P1V1γ = P2V2γ
(c) Work done by the gas in the process,
Q.18. Air (γ = 1.4) is pumped at 2 atm pressure in a motor tyre at 20°C. If the tyre suddenly bursts, what would be the temperature of the air coming out of the tyre? Neglect any mixing with the atmospheric air.
Given:
For air, γ = 1.4
Initial temperature of air, T1 = 20°C = 293 K
Initial pressure, P1 = 2 atm
Final pressure, P2 = 1 atm
The bursting of the tyre is an adiabatic process. For an adiabatic process,
P11-γ × T11-γ = P 1-γ × T2γ
(2)1-1.4 × (293)1.4 = (1) 1-1.4 × T21.4
⇒ (2)-0.4 ×(293)1.4 = T21.4
⇒ 2153.78 =T21.4
⇒ T2 =( 2153.78)1/1.4
= 240.3K
Q.19. A gas is enclosed in a cylindrical can fitted with a piston. The walls of the can and the piston are adiabatic. The initial pressure, volume and temperature of the gas are 100 kPa, 400 cm3 and 300 K, respectively. The ratio of the specific heat capacities of the gas, Cp / Cv = 1.5. Find the pressure and the temperature of the gas if it is (a) suddenly compressed (b) slowly compressed to 100 cm3.
Initial pressure of the gas, P1 = 100 kPa
Initial volume of the gas,V1 = 400 cm3 = 400 × 10−6 m3
Initial temperature of the gas, T1 = 300 K
(a) The gas is suddenly compressed to volume, V2 = 100 cm3 .
So, this is an adiabatic process.
For an adiabatic process,
P1V1γ = P2V2γ
⇒ 105 × (400)1.5 = P2 (100)1.5
⇒ P2 =105 (4)1.5 = 800 kPa
Also,
T1Vγ−1 = T2V2γ−1
⇒ 300 × (400)1.5−1 = T2 (100)1.5−1
⇒ 300 × (400)0.5 = T2 (100)0.5
⇒ T2 = 600 K
(b) If the container is slowly compressed, the heat transfer is zero, even thought the walls are adiabatic.
Thus, the values remain same. Thus,
P2 = 800 kPa
T2 = 600 K
Q.20. The initial pressure and volume of a given mass of a gas (Cp/Cv = γ) are p0 and V0. The gas can exchange heat with the surrounding. (a) It is slowly compressed to a volume V0/2 and then suddenly compressed to V0/4. Find the final pressure. (b) If the gas is suddenly compressed from the volume V0 to V0/2 and then slowly compressed to V0/4, what will be the final pressure?
Given:
For the gas,
Initial pressure of the gas = P0
Initial volume of the gas = V0
(a)
(i) As the gas is slowly compressed, its temperature will remain constant.
For isothermal compression,
P1V1 = P2V2
So, P0V0 =P2
(ii) Sudden compression means that the gas could not get sufficient time to exchange heat with its surroundings. So, it is an adiabatiac compression.
So, for adiabatic compression,
(b) (i) Adiabatic compression:
(ii) Isothermal compression: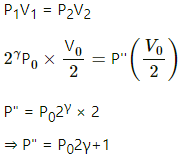
Q.21. Consider a given sample of an ideal gas (Cp/Cv = γ) having initial pressure p0 and volume V0. (a) The gas is isothermally taken to a pressure p0/2 and from there, adiabatically to a pressure p0/4. Find the final volume. (b) The gas is brought back to its initial state. It is adiabatically taken to a pressure p0/2 and from there, isothermally to a pressure p0/4. Find the final volume.
(a) Given,
Initial pressure of the gas = p0
Initial volume of the gas =V0
For an isothermal process,
PV = constant
So, P1V1 = P2V2
For an adiabatic process ,P2V2γ = P3V3γ
Again, for an isothermal process,
Q.22. A sample of an ideal gas (γ = 1.5) is compressed adiabatically from a volume of 150 cm3 to 50 cm3. The initial pressure and the initial temperature are 150 kPa and 300 K. Find (a) the number of moles of the gas in the sample (b) the molar heat capacity at constant volume (c) the final pressure and temperature (d) the work done by the gas in the process and (e) the change in internal energy of the gas.
The ideal gas equation is
PV = nRT
Given, P1 = 150 kPa = 150 × 103 Pa
V1 = 150 cm3 = 150 × 10−6 m3
T1= 300 K
(a)
n = 0.009
(b)
(c) Given,
P1 = 150 kPa = 150 × 103 Pa
P2 = ? V1 = 150 cm3
= 150 × 10−6 m3
γ = 1.5
V2 = 50 cm3 = 50 × 10−6 m3,
T1 = 300 K
T2 = ?
Since the process is adiabatic, using the equation of an adiabatic process,we get
P1V1γ = P2V2γ
⇒ 150 × 103 × (150 × 10−6)γ = P2 × (50 × 10−6)γ
P2 = 150000 × (3)1.5
P2 = 779.422 × 103 Pa
P2 = 780 kPa
Again,
P11−γ T1γ = P11−γ T2γ
⇒ (150 × 103)1−1.5 × (330)1.5 = (780 × 103)1−1.5 × T21.5
⇒ T21.5 = (150 × 103)1−1.5 × (300)1.5 × 3001.5
T21.5 = 11849.050
⇒ T2 = (11849.050)1/1.5
T2 = 519.74 = 520 K
d) dQ = dW + dU
Or dW = −dU [ Since dQ = 0 in an adiabatic process]
dW = −nCvdT
dW = −0.009 × 16.6 × (520 − 300)
dW = −0.009 × 16.6 × 220
dW = −32.87 J ≈ −33 J
(e) dU = nCvdT
dU = 0.009 × 16.6 × 220 ≈ 33 J
Q.23. Three samples A, B and C of the same gas (γ = 1.5) have equal volumes and temperatures. The volume of each sample is doubled, the process being isothermal for A, adiabatic for B and isobaric for C. If the final pressures are equal for the three samples, find the ratio of the initial pressures.
Here are three gases A, B and C.
It is given that initially,
VA = VB = VC and TA = TB = TC .
For A, the process is isothermal and for an isothermal process, PV = constant.
PAVA = P'A2VA
For B, the process is adiabatic. So,
For C, the process is isobaric, which implies that the pressure will remain constant.
So, using the ideal gas equation
As the final pressures are equal,
Q.24. Two samples A and B, of the same gas have equal volumes and pressures. The gas in sample A is expanded isothermally to double its volume and the gas in B is expanded adiabatically to double its volume. If the work done by the gas is the same for the two cases, show that γ satisfies the equation 1 − 21−γ = (γ − 1) ln2.
Let,
Initial pressure of the gas = P1
Initial volume of the gas = V1
Final pressure of the gas= P2
Final volume of the gas = V2
Given, V2 = 2 V1, for each case.
In an isothermal expansion process,
Adiabatic work done,
It is given that same work is done in both cases.
In an adiabatic process,
Q.25. 1 litre of an ideal gas (γ = 1.5) at 300 K is suddenly compressed to half its original volume. (a) Find the ratio of the final pressure to the initial pressure. (b) If the original pressure is 100 kPa, find the work done by the gas in the process. (c) What is the change in internal energy? (d) What is the final temperature? (e) The gas is now cooled to 300 K keeping its pressure constant. Calculate the work done during the process. (f) The gas is now expanded isothermally to achieve its original volume of 1 litre. Calculate the work done by the gas. (g) Calculate the total work done in the cycle.
Given:
γ = 1.5
T = 300 K
Initial volume of the gas, V1 = 1 L
Final volume, V2 = (1/2)L
(a) The process is adiabatic because volume is suddenly changed; so, no heat exchange is allowed.
Work done by an adiabatic process,
W = -82 J
(c) Internal energy,
dQ = 0, as it is an adiabatic process.
⇒ dU = − dW = − (− 82 J) = 82 J
(d) Also, for an adiabatic process,
(e) The pressure is kept constant.
The process is isobaric; so, work done = PΔV=nRdT.
As pressure is constant,
(f) Work done in an isothermal process,
= 100 × ln 2 = 100 × 1.039
= 103 J
(g) Net work done (using first law of thermodynamics)
= − 82 − 41.4 + 103
= − 20.4 J
Q.26. Figure shows a cylindrical tube with adiabatic walls and fitted with an adiabatic separator. The separator can be slid into the tube by an external mechanism. An ideal gas (γ = 1.5) is injected in the two sides at equal pressures and temperatures. The separator remains in equilibrium at the middle. It is now slid to a position where it divides the tube in the ratio 1 : 3. Find the ratio of the temperatures in the two parts of the vessel.
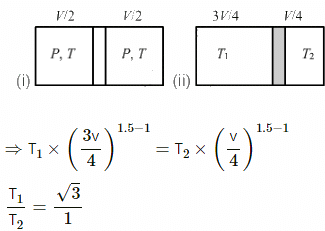
Given:
γ = 1.5
For an adiabatic process, TVγ−1 = constant.
So, T1 V1γ−1 = T2 V2γ−1
As it is an adiabatic process and all the other conditions are same, the above equation can be applied.
In the new position, the slid is dividing the tube in the ratio 3:1.
So, if the total volume is V, then one side will occupy a volume of (3/4)V and the other side will occupy V/4.
Q.27. Figure shows two rigid vessels A and B, each of volume 200 cm3, containing an ideal gas (Cv = 12.5 J K−1 mol−1). The vessels are connected to a manometer tube containing mercury. The pressure in both the vessels is 75 cm of mercury and the temperature is 300 K. (a) Find the number of moles of the gas in each vessel. (b) 5.0 J of heat is supplied to the gas in vessel A and 10 J to the gas in vessel B. Assuming there's no appreciable transfer of heat from A to B, calculate the difference in the heights of mercury in the two sides of the manometer. Gas constant, R = 8.3 J K−1 mol−1.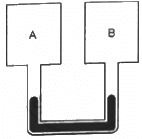
Given:
Volume of gas in each vessel, V = 200 cm3
Specific heat at constant volume of the gas, Cv = 12.5 J/mol-K
Initial temperature of the gas, T = 300 K
Initial pressure of the gas, P = 75 cm of Hg
(a) Using the ideal gas equation, number of moles of gases in each vessel,
75 cm of Hg = 99991.5 N/m2
= 8031.4 x 10-6
=0.008
(b) Heat is supplied to the gas, but dV is zero as the container has rigid walls.
So, dW = P Δ V = 0
From first law of thermodynamics,
dQ = dU
⇒ 5 = nCvdT
⇒ 5 = 0.008 × 12.5 × dT
⇒ dT = 50 for Abecause volume is kept constant.
Q = nCvdT
PB = 25 cm of Hg
The distance moved by the mercury, PB − PA = 25 − 12.5 = 12.5 cm
Q.28. The figure shows two vessels with adiabatic walls, one containing 0.1 g of helium (γ = 1.67, M = 4 g mol−1) and the other containing some amount of hydrogen (γ = 1.4, M = 2 g mol−1). Initially, the temperatures of the two gases are equal. The gases are electrically heated for some time during which equal amounts of heat are given to the two gases. It is found that the temperatures rise through the same amount in the two vessels. Calculate the mass of hydrogen.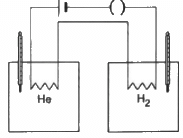
Given:
Mass of He, mHe = 0.1 g
γ1 = 1.67
Molecular weight of He, MHe = 4 g/mol
MH2 = ?
MH2 = 2 g/mol
γ2 = 1.4
Since it is an adiabatic environment and the system is not dong any external work, the amount of heat given will be used up entirely to raise its internal energy.
For He, dQ = dU = nCvdT ...(i)
where m is the required mass of H2.
Since equal amount of heat is given to both gases; so dQ is same in both eq (i) and (ii), we get
Q.29. Two vessels A and B of equal volume V0 are connected by a narrow tube that can be closed by a valve. The vessels are fitted with pistons that can be moved to change the volumes. Initially, the valve is open and the vessels contain an ideal gas (Cp/Cv = γ) at atmospheric pressure p0 and atmospheric temperature T0. The walls of vessel A are diathermic and those of B are adiabatic. The valve is now closed and the pistons are slowly pulled out to increase the volumes of the vessels to double the original value. (a) Find the temperatures and pressures in the two vessels. (b) The valve is now opened for sufficient time so that the gases acquire a common temperature and pressure. Find the new values of the temperature and pressure.
Initial pressure of the gas in both the vessels = P0
Initial temperature of the gas in both the vessels = T0
Initial volume = V0
(a) The temperature inside the diathermic vessel remains constant. Thus,
P1V1 = P2V2
⇒ P0V0 = P2 × 2V0
Temperature = T0
The temperature inside the adiabatic vessel does not remain constant.
So, for an adiabatic process,
(b) When the valves are open, the temperature remains T0 throughout, i.e. T1 = T2 = T0 .
Also, pressure will be same throughout. Thus, P1 = P2. As, temperature has not changed on side 1, so pressure on this side will also not change (volume is also fixed due to fixed piston) and will be equal to P0 (pressure is an intrinsic variable).
On side 2, pressure will change to accommodate the changes in temperature on this side.
So, P0 = P1 + P2
⇒ 2P1 = 2P2
So, P1 = P2 = P0/2
Q.30. The figure shows an adiabatic cylindrical tube of volume V0 divided in two parts by a frictionless adiabatic separator. Initially, the separator is kept in the middle, an ideal gas at pressure p1 and temperature T1 is injected into the left part and another ideal gas at pressure p2 and temperature T2 is injected into the right part. Cp/Cv = γ is the same for both the gases. The separator is slid slowly and is released at a position where it can stay in equilibrium. Find (a) the volumes of the two parts (b) the heat given to the gas in the left part and (c) the final common pressure of the gases.
For an adiabatic process, PVγ = Constant
So, P1V1γ = P2V2γ ...(i)
According to the problem,
V1 + V2 = V0 ...(ii)
Using the relation in eq (ii) in eq (i), we get
P1V1γ = P2(V0 − V1)γ
Using equation (ii), we get
(b) Since the whole process takes place in adiabatic surroundings, the separator is adiabatic.
Hence, heat given to the gas in the left part is 0.
(c) There will be a common pressure 'P' when equilibrium is reached. The slid will move until the pressure on the two sides becomes equal.
P1V1γ + P2V2γ = PV0γ
Q.31. An adiabatic cylindrical tube of cross-sectional area 1 cm2 is closed at one end and fitted with a piston at the other end. The tube contains 0.03 g of an ideal gas. At 1 atm pressure and at the temperature of the surrounding, the length of the gas column is 40 cm. The piston is suddenly pulled out to double the length of the column. The pressure of the gas falls to 0.355 atm. Find the speed of sound in the gas at atmospheric temperature.
Given:
Area of the tube, A = 1 cm2 = 1 × 10−4 m2
Mass of the gas, M = 0.03 g = 0.03 × 10−3 kg
Initial pressure, P = 1 atm = 105 Pascal
Initial length of the mercury column, L = 40 cm = 0.4 m
Final length of the mercury column, L1 = 80 cm = 0.8 m
Final pressure, P' = 0.355 atm
The process is adiabatic. So,
P(V)γ = P'(V')γ
⇒ 1 × (A × 0.4)γ = 0.355 × (A × 0.8)γ
⇒ 1 × 1 = 0.355 × 2γ
Speed of sound in the gas,
⇒ v = 446.33 ≈ 447 m/s
Q.32. The speed of sound in hydrogen at 0°C is 1280 m s−1. The density of hydrogen at STP is 0.089 kg m−3. Calculate the molar heat capacities Cp and Cv of hydrogen.
Given:
Velocity of sound in hydrogen, V = 1280 m/s
Temperature, T = 0°C = 273 K
Density of H2 = 0.089 kg/m3
R = 8.3 J/mol-K
At STP,
P = 105 Pa
We know:
= 18.0J /mol -K
Cp = γ Cv =1.46 × 18.0
= 26.28 ≈ 26.3 / mol -K
Q.33. 4.0 g of helium occupies 22400 cm3 at STP. The specific heat capacity of helium at constant pressure is 5.0 cal K−1 mol−1. Calculate the speed of sound in helium at STP.
Given:
Specific heat capacity at constant pressure, Cp = 5.0 cal/mol-K
Cp = 5.0 × 4.2 J/mol-K
Cp = 21 J/mol-K
Volume of helium, V = 22400 cm3 = 0.0224 m3
At STP, P = 1 atm = 105 Pa
The speed of sound in gas,
v = 960 m/s
Q.34. An ideal gas of density 1.7 × 10−3 g cm−3 at a pressure of 1.5 × 105 Pa is filled in a Kundt's tube. When the gas is resonated at a frequency of 3.0 kHz, nodes are formed at a separation of 6.0 cm. Calculate the molar heat capacities Cp and Cv of the gas.
Given:
Density of the ideal gas, ρ = 1.7 × 10−3 g/cm3
= 1.7 k/gm3
Pressure of the gas, P = 1.5 × 105 Pa
R = 8.3 J/mol-K
Resonance frequency of the gas = 3.0 kHz
Node separation in the Kundt's tube
So, l = 2×6 = 12 cm = 12 × 10−2 m
So, V = fl = 3 × 103 × 12 × 10−2
= 360 m/s
Speed of sound,
= 17.7 J / mol -K
Cp = R +Cv =8.3 +17.7 = 26 J /mol -K
Q.35. Standing waves of frequency 5.0 kHz are produced in a tube filled with oxygen at 300 K. The separation between the consecutive nodes is 3.3 cm. Calculate the specific heat capacities Cp and Cv of the gas.
Frequency of standing waves, f = 5 × 103 Hz
Temperature of oxygen, T = 300 K
From Kundt's tube theory, we know that I/2= node separation = 3.3 cm
∴ l = 6.6 × 10−2 m
Also,
v = fl = 5 × 103 × 6.6 × 10−2
= (66 × 5) = 330 m/s
Specific heat at constant volume,
= 20.7 J mol -K
Specific heat at constant pressure, Cp = Cv + R
Cp = 20.7 + 8.3
Cp = 29.0 J/mol-K
|
134 docs
|