Heat Transfer | Physics Class 11 - NEET PDF Download
| Table of contents |

|
| Modes of Heat Transfer |

|
| What is Conduction? |

|
| Convection |

|
| Formation of Trade Winds |

|
| Radiation |

|
| Prevost Theory of Exchange |

|
| Stefan-Boltzmann's Law |

|
| Summary |

|
In our daily life, we note that a utensil placed on fire becomes hot. When one end of the metal is heated, the other gets heated up after some time. When you sit near a bonfire you start feeling warm. What is the cause of these facts?

Modes of Heat Transfer
The movement of heat across the border of the system is due to a difference in temperature between the system and its surroundings. Heat always flows from the higher temperature side to the lower temperature side.
There are three modes of heat transfer:
- Conduction
- Convection
- Radiation

Here is the difference between the three modes of Heat Transfer:

What is Conduction?
Heat conduction is the transfer of thermal energy (heat) through direct contact between particles in a material. It occurs in solids, liquids, and gases, but it is most effective in solids because the particles are closely packed.
 Conduction
Conduction
In the figure above area of higher kinetic energy i.e. the end that is in contact with flame transfers thermal energy towards the lower kinetic energy area i.e. the other end. High-speed particles clash with particles moving at a slow speed, as a result, slow-speed particles increase their kinetic energy.
This is a typical form of heat transfer and takes place through physical contact. When we hold ice cubes in our hands, heat is transferred from our hands to the ice cube resulting in the melting of ice cubes. This also happens due to conduction.
- The figure below shows a rod whose ends are in thermal contact with a hot reservoir at temperature T1 and a cold reservoir at temperature T2. The sides of the rod are covered with an insulating medium, so the transport of heat is along the rod, not through the sides.
- The molecules in the hot reservoir have greater vibrational energy. This energy is transferred by collisions to the atoms at the end face of the rod.
- These atoms in turn transfer energy to their neighbors further along the rod. Such transfer of heat through a substance in which heat is transported without direct mass transport is called conduction.

- Most metals use another, more effective mechanism to conduct heat. The free electrons, which move throughout the metal can rapidly carry energy from the hotter to cooler regions, so metals are generally good conductors of heat.
- The presence of 'free' electrons also causes most metals to be good electrical conductors.
- A metal rod at 5°C feels colder than a piece of wood at 5°C because heat can flow more easily from your hand into the metal.
- Heat transfer occurs only between regions that are at different temperatures, and the rate of heat flow is
. This rate is also called the heat current, denoted by H.
- Experiments show that the heat current is proportional to the cross-section area A of the rod and to the temperature gradient
, which is the rate of change of temperature with distance along the bar. In general
- The negative sign is used to make
a positive quantity since
is negative.
- The constant k, called the thermal conductivity is a measure of the ability of a material to conduct heat.
- A substance with a large thermal conductivity is a good heat conductor. The value of k depends on the temperature, increasing slightly with increasing temperature, but k can be taken to be practically constant throughout a substance if the temperature difference between its ends is not too great.
Let us apply Eq....(i) to a rod of length L and constant cross-sectional area A in which a steady state has been reached. In a steady state, the temperature at each point is constant in time. Hence.

Therefore, the heat DQ transferred in time dt is
Here, dT = temperature difference (TD) and = thermal resistance of the rod.
Convection
Convection is the process of heat transfer in fluids by the actual motion of molecules in the fluids. It happens in liquids as well as gases. It involves a bulk transfer of portions of the fluid.
- Heating a fluid from beneath triggers thermal expansion, causing the warmer lower layers to decrease in density. It's important to note that colder fluid is denser.
- Consequently, buoyancy forces the less dense, hotter portion of the fluid to ascend, while the colder, denser fluid descends to take its place.
- This cycle continues as the heated portion rises and is replaced by the colder upper layer, illustrating the mechanism by which heat is transferred via convection.
Land and Sea Breezes
During the day, the land heats up faster than the sea. This occurs because the water has greater specific heat and because mixing currents disperse the absorbed heat throughout the great volume of water.
The hot air above the land expands and becoming less dense and hence the warmer air rises and colder air from the sea takes its place. The warmer air from the land moves towards the sea to complete the cycle. This create a breeze from the sea to the land which is called a sea breeze.
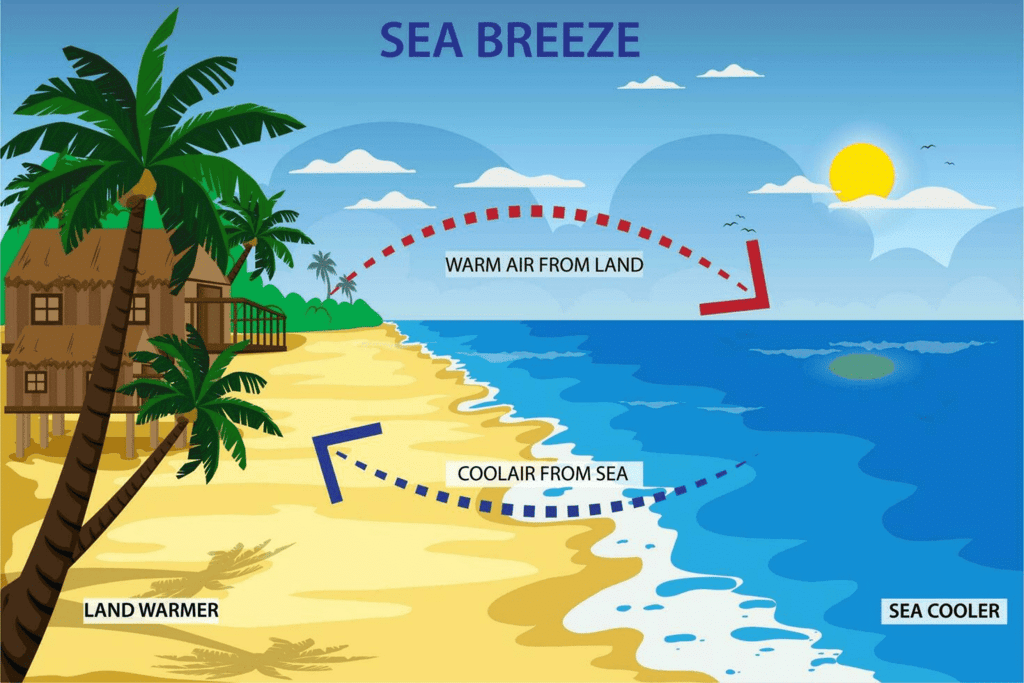 Sea Breeze
Sea Breeze
During the night, the opposite happens. The land cools faster than sea. The warm air above the sea rises. This warm air is replaced by colder air from the land creating a land breeze.
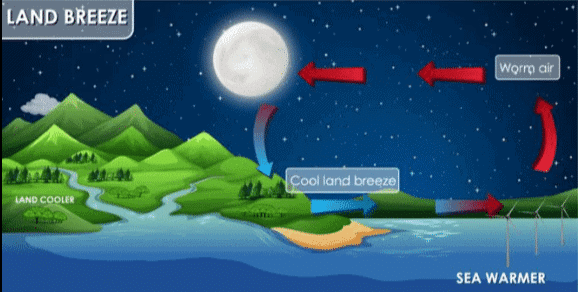 Land Breeze
Land Breeze
Formation of Trade Winds
- The equatorial and polar regions of the Earth receive different amounts of solar energy.
- At the equator, the air close to the Earth's surface becomes warm, while the air in the upper atmosphere near the poles remains cool.
- This temperature difference creates a convection current between two regions.
- The warm air at the equator rises and moves toward the poles, where it cools, descends, and flows back toward the equator.
- The Earth's rotation affects this current, as the moving air at the equator has an eastward velocity of 1600 km/h.
- The air closer to the poles has little to no eastward speed, so it doesn’t descend at the poles.
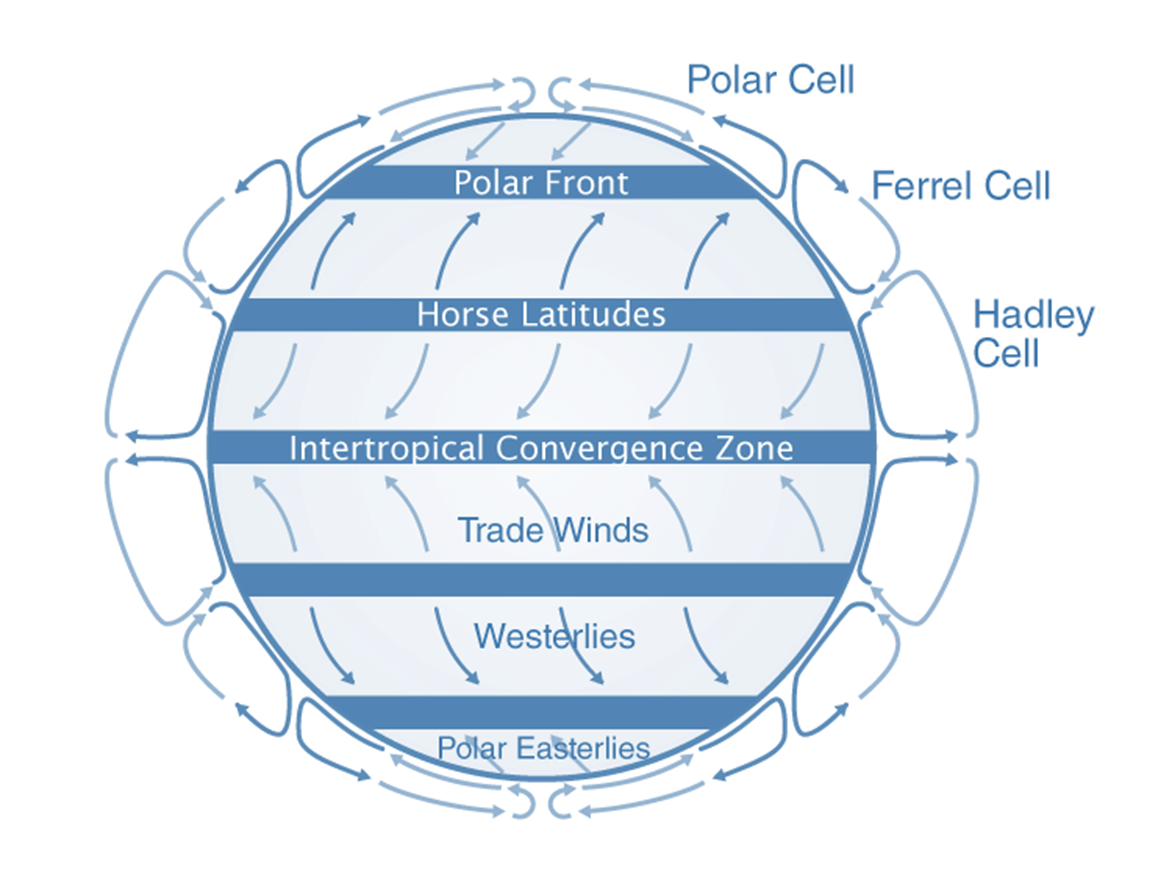 Trade Winds
Trade Winds
- Instead, it descends at about 30° latitude north and south and moves back toward the equator.
- This circulation pattern is known as the trade wind.
- Thus, the continuous flow of air from the northeast toward the equator, close to the Earth’s surface, is called a trade wind, which is an example of natural convection.
Radiation
The process of the transfer of heat from one place to another place without heating the intervening medium is called radiation.
The term radiation used here is another word for electromagnetic waves. These waves are formed due to the superposition of electric and magnetic fields perpendicular to each other and carry energy.
Properties of Radiation
- All objects emit radiation simply because their temperature is above absolute zero, and all objects absorb some of the radiation that falls on them from other objects.
- Maxwell on the basis of his electromagnetic theory proved that all radiations are electromagnetic waves and their sources are vibrations of charged particles in atoms and molecules.
- More radiation is emitted at higher temperatures of a body and less at lower temperatures.
- The wavelength corresponding to the maximum emission of radiation shifts from a longer wavelength to a shorter wavelength as the temperature increases. Due to this, the color of a body appears to be changing. Radiations from a body at NTP has predominantly infrared waves.
- Thermal radiation travels with the speed of light and moves in a straight line.
- Radiations are electromagnetic waves and can also travel through a vacuum.
- Similar to light, thermal radiations can be reflected, refracted, diffracted, and polarized.
- Radiation from a point source obeys inverse square law (intensity a
)
Prevost Theory of Exchange
- According to this theory, all bodies radiate thermal radiation at all temperatures. The amount of thermal radiation radiated per unit of time depends on the nature of the emitting surface, its area, and its temperature.
- The rate is faster at higher temperatures. Besides, a body also absorbs part of the thermal radiation emitted by the surrounding bodies when this radiation falls on it. If a body radiates more than what it absorbs, its temperature falls.
- If a body radiates less than what it absorbs, its temperature rises. And if the temperature of a body is equal to the temperature of its surroundings it radiates at the same rate as it absorbs.
Perfectly Black Body and Black Body Radiation (Fery's Black Body)
- A perfectly black body is one which absorbs all the heat radiations of whatever wavelength, is incident on it. It neither reflects nor transmits any of the incident radiation and therefore appears black whatever the color of the incident radiation.
- In actual practice, no natural object possesses strictly the properties of a perfectly black body. But the lamp-black and platinum black are a good approximation of the black body. They absorb about 99% of the incident radiation.
- The most simple and commonly used black body was designed by Fery. It consists of an enclosure with a small opening which is painted black from inside.
- The opening acts as a perfect black body. Any radiation that falls on the opening goes inside and has very little chance of escaping the enclosure before getting absorbed through multiple reflections.
- The cone opposite to the opening ensures that no radiation is reflected back directly.

Absorption, Reflection, and Emission of Radiations
where r = reflecting power, a = absorptive power and t = transmission power.
(i) r = 0, t = 0, a = 1, perfect black body
(ii) r = 1, t = 0, a = 0, perfect reflector
(iii) r = 0, t = 1, a = 0, perfect transmitter
(a) Absorptive Power
In particular, absorptive power of a body can be defined as the fraction of incident radiation that is absorbed by the body.
a =
As all the radiations incident on a black body are absorbed, a = 1 for a black body.
(b) Emissive Power
- Consider a small area DA of a body emitting thermal radiation.
- Consider a small solid angle Dw about the normal to the radiating surface.
- Let the energy radiated by the area DA of the surface in the solid angle Dw in time Dt be DU.
- We define emissive power of the body as

Thus, emissive power denotes the energy radiated per unit area per unit time per unit solid angle along the normal to the area.
(c) Spectral Emissive Power (El)
Emissive power per unit wavelength range at wavelength l is known as spectral emissive power, El. If E is the total emissive power and El is the spectral emissive power, they are related as follows,
E = and
(d) Emissivity
e = =
Stefan-Boltzmann's Law
Consider a hot body at temperature T placed in an environment at a lower temperature T0. The body emits more radiation than it absorbs and cools down while the surroundings absorb radiation from the body and warm up. The body is losing energy by emitting radiation and this rate.

and is receiving energy by absorbing radiation and this absorption rate
=
Here, 'a' is a pure number between 0 and 1 indicating the relative ability of the surface to absorb radiation from its surroundings. Note that this 'a' is different from the absorptive power 'a'. In thermal equilibrium, both the body and the surroundings have the same temperature (say Tc), and,
P1 = P2
or
or e = a
Thus, when T > T0, the net rate of heat transfer from the body to the surroundings is,
Net heat loss =
or ⇒ Rate of cooling
or
Wein's Displacement Law
Wien’s Displacement Law states that the wavelength (λm) at which a black body emits energy most intensely is inversely related to its absolute temperature (T). In other words, as the temperature increases, the peak wavelength of emitted energy decreases.
Mathematically, this is expressed as:

Summary
- The equation
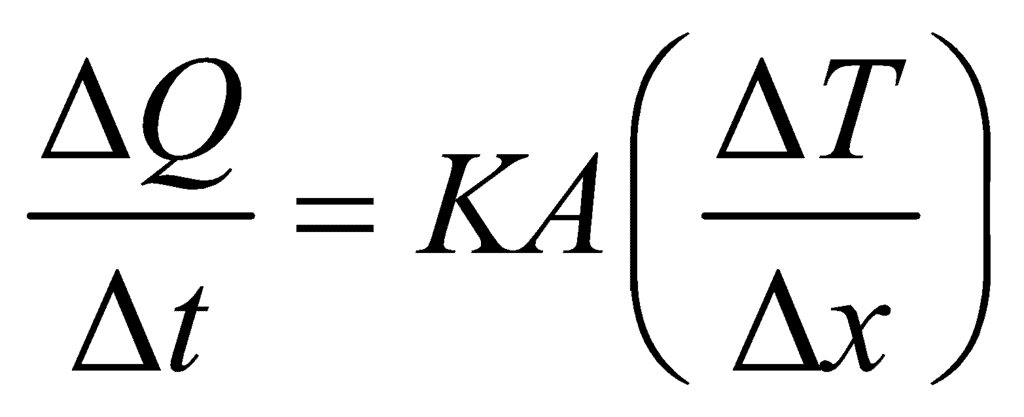 is valid for steady-state conditions. The condition is said to occur when no part of the heat is used up in raising the temperature of any part of the cross-section of the solid.
is valid for steady-state conditions. The condition is said to occur when no part of the heat is used up in raising the temperature of any part of the cross-section of the solid. - On comparing equation (1) with the following equation used for flowing of charge on account of potential difference.
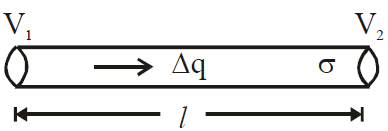


- Series combination of conductors
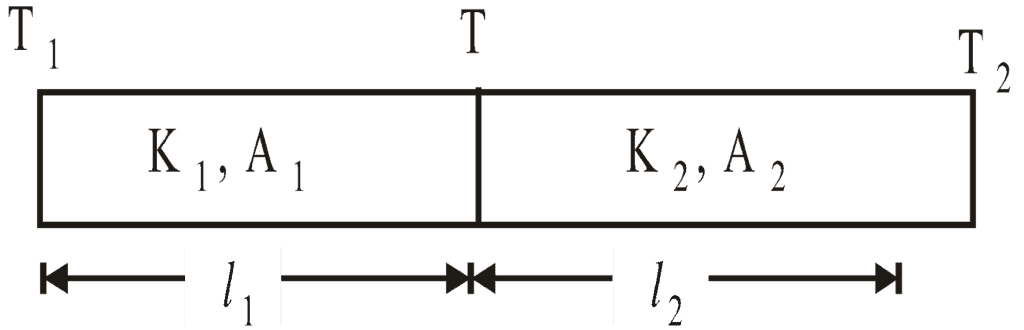
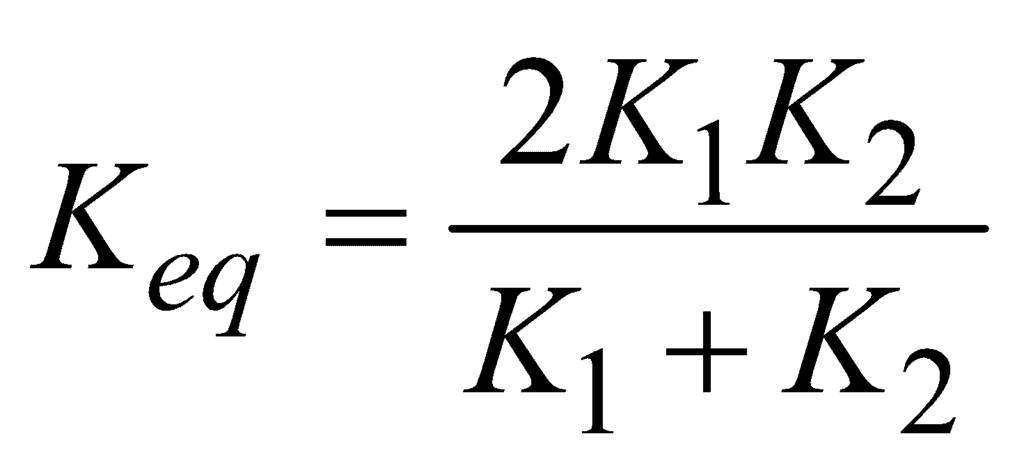
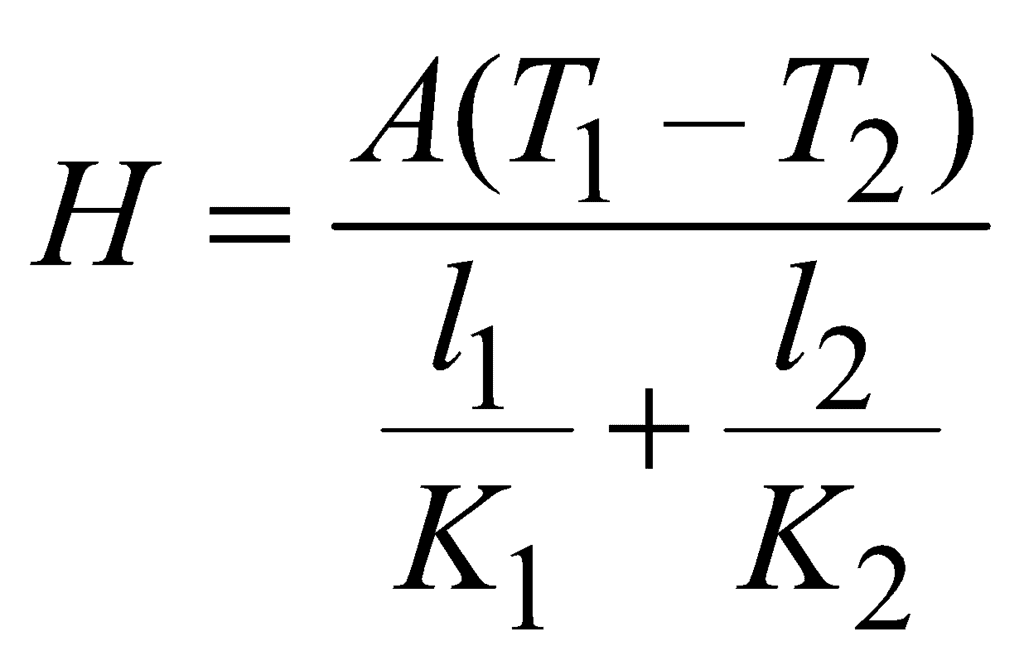
- Parallel combination of conductors
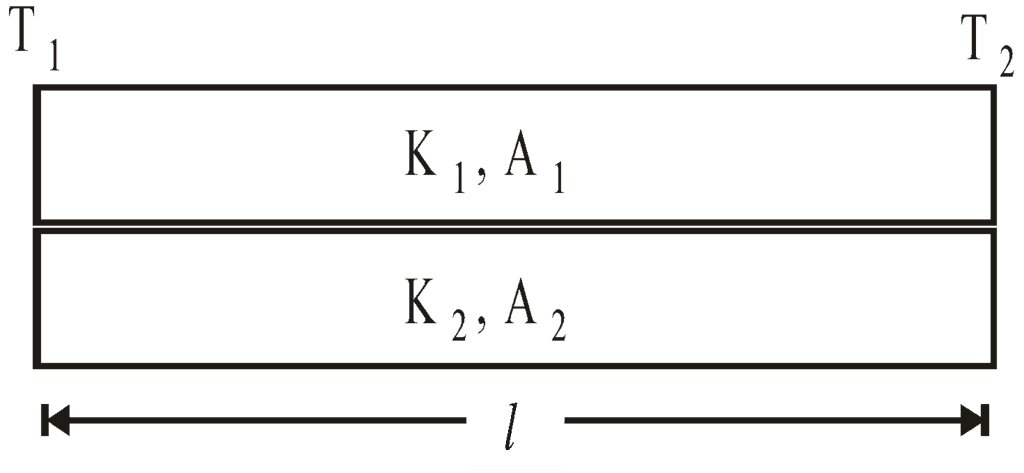

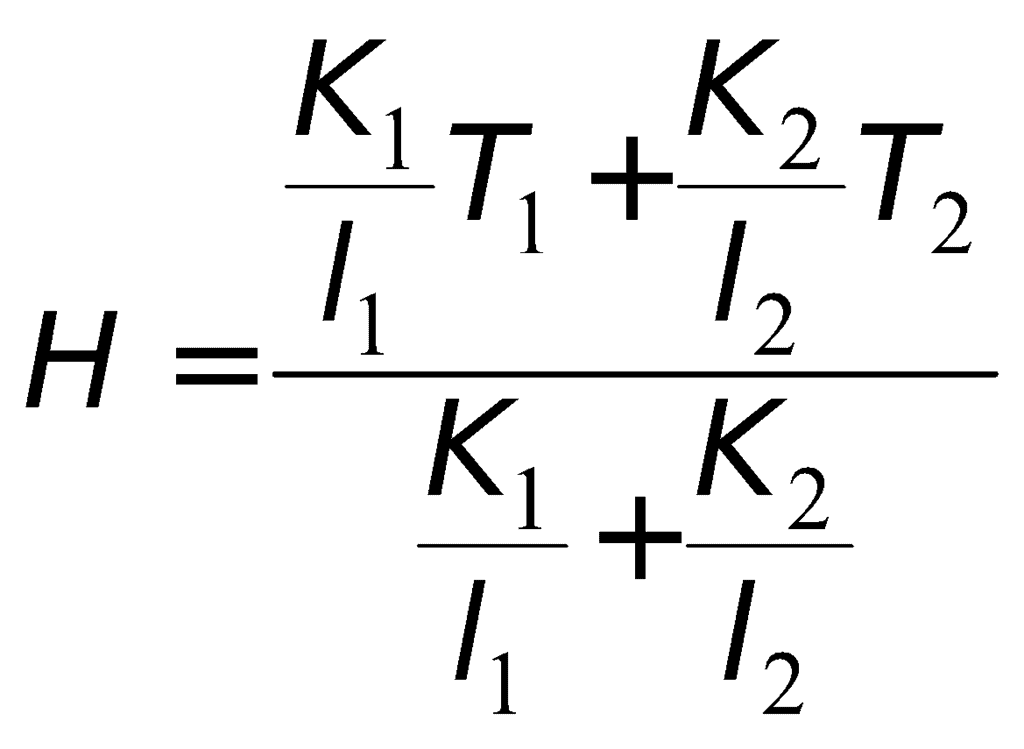
- Davy’s safety lamp is based on conduction. It is used in mines to know the ignition temperature of gases. The danger of explosion can be avoided.
- The principle of chimneys used in a kitchen or a factory is based on convection.
- Land and sea breezes are due to the convection.
- The temperature of the upper part of the flame is more than the temperature on the sides because the currents of air carry the heat upwards.
- Radiation can be detected by differential air thermometer, Bolometer, thermopile, etc.
- A good absorber is a good emitter.
- Cooking utensils are provided with wooden or ebonite handles since wood or ebonite is a bad conductor of heat.
- Good conductors of heat are good conductors of electricity, Mica is an exception which being a good conductor of heat is a bad conductor of electricity.
|
95 videos|367 docs|98 tests
|
FAQs on Heat Transfer - Physics Class 11 - NEET
| 1. What is conduction and how does it occur? |  |
| 2. How does convection differ from conduction? |  |
| 3. What causes the formation of trade winds? |  |
| 4. What is the Prevost Theory of Exchange? |  |
| 5. How is Stefan-Boltzmann's Law related to radiation? |  |
















