Important Diagrams: Is Matter Around Us Pure? | Science Class 9 PDF Download
Q1: Answer the following questions based on the diagram given below:
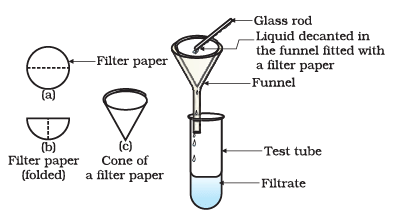
(i) What factors influence the filtration of a solution?
Ans: Various factors affect filtration, including:
- Temperature
- Density
- Pressure
- Viscosity
- Particle size
- Particle shape
- Charge
- Corrosiveness
(ii) Define filtration.
Ans: Filtration is the process of separating insoluble particles from liquids. It uses physical, mechanical, or biological methods to isolate solids from fluids. The resulting liquid is called the filtrate.
(iii) How is filtration performed?
Ans: The basic method of filtration involves:
- Pouring the mixture onto a filter medium (e.g., filter paper).
- Using gravity to pull the liquid down.
- The solid stays on the filter, while the liquid, known as the filtrate, flows through.
(iv) The liquid that passes through the filter paper during filtration is called what?
(a) decantate
(b) filtrate
(c) residue
(d) solute
Answer: (b)
Filtration separates insoluble solids from a liquid or gas. The liquid that passes through the filter paper is referred to as the filtrate.
(v) What is the purpose of filter paper?
Ans: Filter paper serves the purpose of effectively filtering out solid particles from a liquid or gas mixture. It provides a medium for separating solids from fluids during the filtration process.
Q2: Answer the following questions based on the diagram given below: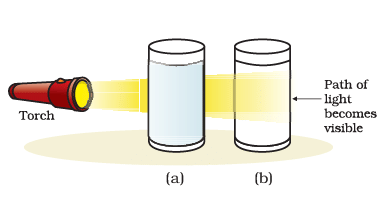 (i) What causes the bluish tinge in the light passed through the mixture of water and milk?
(i) What causes the bluish tinge in the light passed through the mixture of water and milk?
Ans: The bluish tinge is caused by the scattering of light by milk particles, resulting in the Tyndall effect.
(ii) Define the Tyndall effect based on the observation in the experiment.
Ans: The Tyndall effect is the scattering of light by colloidal particles, as observed when light passes through the mixture of water and milk.
(iii) Explain why the solution of copper sulfate does not show the Tyndall effect.
Ans: The solution of copper sulfate does not show the Tyndall effect because the copper sulfate particles are too small to scatter light effectively, unlike the larger milk particles.
(iv) What is the significance of observing the Tyndall effect in the mixture of water and milk?
Ans: The observation of the Tyndall effect in the mixture of water and milk indicates that milk is a colloidal solution, as colloidal particles scatter light, causing the bluish tinge.
(v) Which term best describes the mixture of water and milk based on the experiment?
Ans: The term that best describes the mixture of water and milk based on the experiment is colloidal.
|
85 videos|549 docs|60 tests
|
FAQs on Important Diagrams: Is Matter Around Us Pure? - Science Class 9
| 1. What is the difference between pure substances and mixtures? |  |
| 2. How can we determine if a substance is pure? |  |
| 3. What are examples of homogeneous and heterogeneous mixtures? |  |
| 4. Why is it important to understand the difference between pure substances and mixtures? |  |
| 5. What methods can be used to separate mixtures into pure substances? |  |





















