Structure of Atom Important Notes | Chemistry Class 11 - NEET PDF Download
What is Atomic Structure?
- The atomic structure of an element refers to the constitution of its nucleus and the arrangement of the electrons around it. Primarily, the atomic structure of matter is made up of protons, electrons, and neutrons.
- The protons and neutrons make up the nucleus of the atom, which is surrounded by the electrons belonging to the atom. The atomic number of an element describes the total number of protons in its nucleus.
 Structure of Atom
Structure of Atom - Neutral atoms have equal numbers of protons and electrons. However, atoms may gain or lose electrons to increase their stability and the resulting charged entity is called an ion.
- Atoms of different elements have different atomic structures because they contain different numbers of protons and electrons. This is the reason for the unique characteristics of different elements.
Atomic Models
- The history of atomic structure and quantum mechanics dates back to the times of Democritus, the man who first proposed that matter is composed of atoms.
- The study of the structure of an atom gives a great insight into the entire class of chemical reactions, bonds, and their physical properties. The first scientific theory of atomic structure was proposed by John Dalton in the 1800s.
The advances in atomic structure and quantum mechanics have led to the discovery of other fundamental particles. The discovery of subatomic particles has been the base for many other discoveries and inventions. - In the 18th and 19th centuries, many scientists attempted to explain the structure of the atom with the help of atomic models. Each of these models had its own merits and demerits and was pivotal to the development of the modern atomic model.
- The most notable contributions to the field were by the scientists John Dalton, J.J. Thomson, Ernest Rutherford, and Niels Bohr. Their ideas on the structure of the atom are discussed in this subsection.
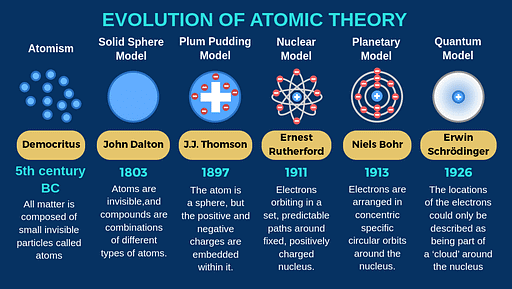 Evolution of Atomic Theory
Evolution of Atomic Theory
Dalton’s Atomic Theory
- The English chemist John Dalton suggested that all matter is made up of atoms, which were indivisible and indestructible. He also stated that all the atoms of an element were the same, but the atoms of different elements differ in size and mass.
- Chemical reactions, according to Dalton’s atomic theory, involve a rearrangement of atoms to form products. According to the postulates proposed by Dalton, the atomic structure comprises atoms, the smallest particles responsible for the chemical reactions to occur.
The following are the postulates of his theory:
- Every matter is made up of atoms.
- Atoms are indivisible.
- Specific elements have only one type of atom in them.
- Each atom has its constant mass that varies from element to element.
- Atoms undergo rearrangement during a chemical reaction.
- Atoms can neither be created nor destroyed but can be transformed from one form to another.
Dalton’s atomic theory successfully explained the Laws of chemical reactions, namely, the Law of conservation of mass, the Law of constant properties, the Law of multiple proportions, and the Law of reciprocal proportions.
Demerits of Dalton’s Atomic Theory
- The theory was unable to explain the existence of isotopes.
- Nothing about the structure of the atom was appropriately explained.
- Later, the scientists discovered particles inside the atom that proved, that the atoms are divisible.
The discovery of particles inside atoms led to a better understanding of chemical species, these particles inside the atoms are called subatomic particles. The discovery of various subatomic particles is as follows:
Thomson Atomic Model
The English chemist Sir Joseph John Thomson put forth his model describing the atomic structure in the early 1900s. He was later awarded the Nobel Prize for the discovery of “electrons”. His work is based on an experiment called the cathode ray experiment. The construction of working of the experiment is as follows:
Cathode Ray Experiment
It has a tube made of glass which has two openings, one for the vacuum pump and the other for the inlet through which a gas is pumped in.
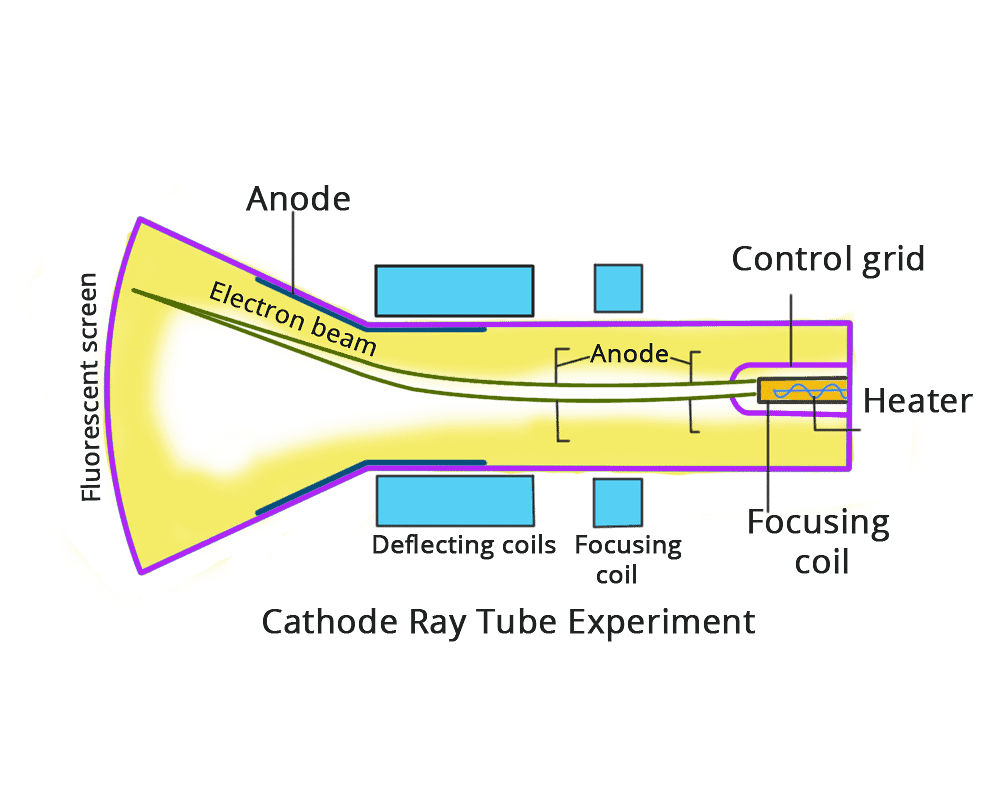 Cathode Ray Tube Experiment
Cathode Ray Tube Experiment
The role of the vacuum pump is to maintain a “partial vacuum” inside the glass chamber. A high voltage power supply is connected using electrodes i.e. cathode and Anode is fitted inside the glass tube.
Observations
- When a high-voltage power supply is switched on, rays emerge from the cathode towards the anode. This was confirmed by the ‘Fluorescent spots’ on the ZnS screen used. These rays were called “Cathode Rays”.
- When an external electric field is applied, the cathode rays get deflected toward the positive electrode, but in the absence of an electric field, they travel in a straight line.
- When rotor Blades are placed in the path of the cathode rays, they seem to rotate. This proves that the cathode rays are made up of particles of a certain mass so that they have some energy.
- With all this evidence, Thompson concluded that cathode rays are made of negatively charged particles called “electrons”.
- On applying the electric and magnetic field upon the cathode rays (electrons), Thomson found the charge-to-mass ratio (e/m) of electrons. (e/m) for electron: 17588 × 1011 e/bg.
From this ratio, the charge of the electron was found by Mullikan through an oil drop experiment. [Charge of e– = 1.6 × 10-16 C and Mass of e– = 9.1093 × 10-31 kg].
Conclusions
- Based on conclusions from his cathode ray experiment, Thomson described the atomic structure as a positively charged sphere into which negatively charged electrons were embedded.
- It is commonly referred to as the “plum pudding model” because it can be visualized as a plum pudding dish where the pudding describes the positively charged atom and the plum pieces describe the electrons.
- Thomson’s atomic structure described atoms as electrically neutral, i.e. the positive and the negative charges were of equal magnitude.
- Limitations of Thomson’s Atomic Structure: Thomson’s atomic model does not clearly explain the stability of an atom. Also, further discoveries of other subatomic particles, couldn’t be placed inside his atomic model.
Rutherford Atomic Theory
Rutherford, a student of J. J. Thomson modified the atomic structure with the discovery of another subatomic particle called “Nucleus”. His atomic model is based on the Alpha ray scattering experiment.
Alpha Ray Scattering Experiment
Construction
- A very thin gold foil of 1000 atoms thick is taken.
- Alpha rays (doubly charged Helium He2+) were made to bombard the gold foil.
- Zn S screen is placed behind the gold foil.
Observations
- Most of the rays just went through the gold foil making scintillations (bright spots) in the ZnS screen.
- A few rays got reflected after hitting the gold foil.
- One in 1000 rays got reflected by an angle of 180° (retraced path) after hitting the gold foil.
Conclusions
- Since most rays passed through, Rutherford concluded that most of the space inside the atom is empty.
- Few rays got reflected because of the repulsion of its positive with some other positive charge inside the atom.
- 1/1000th of rays got strongly deflected because of a very strong positive charge in the center of the atom. He called this strong positive charge as “nucleus”.
- He said most of the charge and mass of the atom resides in the Nucleus

Rutherford’s Structure of Atom
Based on the above observations and conclusions, Rutherford proposed his atomic structure which is as follows.
- The nucleus is at the center of an atom, where most of the charge and mass are concentrated.
- The atomic structure is spherical.
- Electrons revolve around the nucleus in a circular orbit, similar to how planets orbit the sun.
Limitations of the Rutherford Atomic Model
- If electrons have to revolve around the nucleus, they will spend energy, and that too against the strong force of attraction from the nucleus, a lot of energy will be spent by the electrons and eventually, they will lose all their energy and fall into the nucleus so the stability of atom is not explained.
- If electrons continuously revolve around the ‘nucleus, the type of spectrum expected is a continuous spectrum. But in reality, what we see is a line spectrum.
Subatomic Particles
Protons
- Protons are positively charged subatomic particles. The charge of a proton is 1e, which corresponds to approximately 1.602 × 10-19
- The mass of a proton is approximately 1.672 × 10-24
- Protons are over 1800 times heavier than electrons.
- The total number of protons in the atoms of an element is always equal to the atomic number of the element.

Neutrons
- The mass of a neutron is almost the same as that of a proton i.e. 1.674×10-24
- Neutrons are electrically neutral particles and carry no charge.
- Different isotopes of an element have the same number of protons but vary in the number of neutrons present in their respective nuclei.
Electrons
- The charge of an electron is -1e, which approximates to -1.602 × 10-19
- The mass of an electron is approximately 9.1 × 10-31.
- Due to the relatively negligible mass of electrons, they are ignored when calculating the mass of an atom.
Atomic Structure of Isotopes
Nucleons are the components of the nucleus of an atom. A nucleon can either be a proton or a neutron. Each element has a unique number of protons in it, which is described by its unique atomic number. However, several atomic structures of an element can exist, which differ in the total number of nucleons.
These variants of elements having a different nucleon number (also known as the mass number) are called isotopes of the element. Therefore, the isotopes of an element have the same number of protons but differ in the number of neutrons.
The atomic structure of an isotope is described with the help of the chemical symbol of the element, the atomic number of the element, and the mass number of the isotope. For example, there exist three known naturally occurring isotopes of hydrogen, namely, protium, deuterium, and tritium. The atomic structures of these hydrogen isotopes are illustrated below.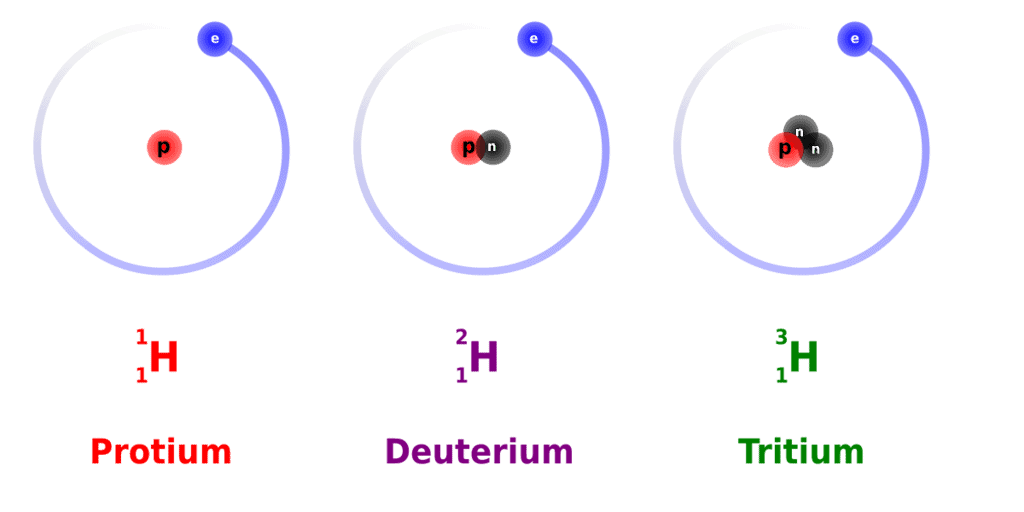 Isotopes of Hydrogen
Isotopes of Hydrogen
The isotopes of an element vary in stability. The half-lives of isotopes also differ. However, they generally have similar chemical behavior because they hold the same electronic structures.
Bohr’s Atomic Theory
Neils Bohr put forth his model of the atom in the year 1915. This is the most widely used atomic model to describe the atomic structure of an element which is based on Planck’s theory of quantization.
Postulates
- The electrons inside atoms are placed in discrete orbits called “stationary orbits”.
- The energy levels of these shells can be represented via quantum numbers.
- Electrons can jump to higher levels by absorbing energy and move to lower energy levels by losing or emitting their energy.
- As long as an electron stays in its stationary orbit, there will be no absorption or emission of energy.
- Electrons revolve around the nucleus in these stationary orbits only.
- The energy of the stationary orbits is quantized.
Limitations of Bohr’s Atomic Theory
- Bohr’s atomic structure works only for single electron species such as H, He+, Li2+, Be3+, ….
- When the emission spectrum of hydrogen was observed under a more accurate spectrometer, each line spectrum was seen to be a combination of no smaller discrete lines.
- Both Stark and Zeeman's effects couldn’t be explained using Bohr’s theory.
- Stark effect: Phenomenon of deflection of electrons in the presence of an electric field.
- Zeeman effect: Phenomenon of deflection of electrons in the presence of a magnetic field.
Planck’s Quantum Theory
To explain 'Black body radiation' and the photoelectric effect, Max Planck proposed Planck’s Quantum Theory in 1900, later extended by Einstein in 1905. The key points of this theory are:
- The radiant energy is emitted or absorbed in the form of small packets known as quanta.
- The energy of each quantum is directly proportional to the frequency of the radiation, where h is Planck’s constant (h = 6.626 x 10-34 Jsec).
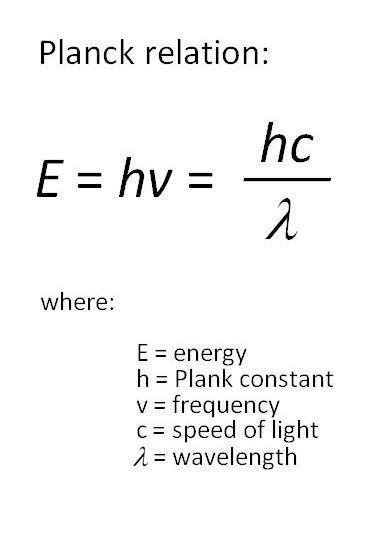 Planck Relation
Planck Relation
Photoelectric Effect
In 1887, Hertz discovered the photoelectric effect, where electrons are emitted from a metal surface when light of a certain frequency strikes it. Observations in the photoelectric effect include:
- Only photons with a minimum frequency (threshold frequency, v0) can cause the photoelectric effect, and this threshold varies for different metals.
- The kinetic energy of emitted electrons is directly proportional to the frequency of striking photons, independent of their intensity.
- The number of ejected electrons depends on the intensity of striking photons, not their frequency.

Explanation of Photoelectric Effect
In 1905, Einstein explained the photoelectric effect using Planck’s quantum theory:
- Photoelectrons are ejected only when the incident light has a minimum frequency (threshold frequency, v0).
- If the frequency of the incident light (v) exceeds the threshold frequency, the excess energy (hv – hv0) imparts kinetic energy to the emitted electrons.
- Increasing light intensity ejects more electrons, but their energies remain unaltered.
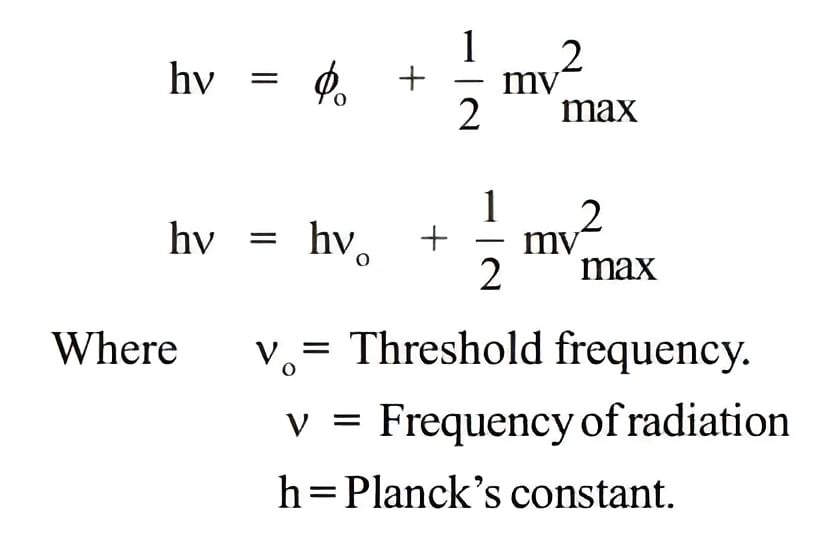 Photoelectric Effect Equation
Photoelectric Effect Equation
Dual Behaviour of Electromagnetic Radiation
From the study of the behavior of light, scientists concluded that light and other electromagnetic radiations exhibit a dual nature – both wave and particle characteristics. When radiation interacts with matter, it displays particle-like properties, while it exhibits wave-like properties such as interference and diffraction during propagation. This wave-particle duality is also observed in microscopic particles like electrons.
Spectrum
When a ray of white light passes through a prism, the wave with a shorter wavelength bends more than the one with a longer wavelength. This dispersion results in a series of colored bands called a spectrum. The continuous spectrum spans from violet to red, resembling a rainbow.
Emission Spectra
Emission spectra occur when radiations emitted from a source, like the sun or a glowing electric bulb, pass through a prism and are received on a photographic plate. Other ways of emitting radiations include passing electric discharge through low-pressure gas or heating a substance to high temperature.
Line Spectra
When vapors of volatile substances fall on the flame of a Bunsen burner and are analyzed with a spectroscope, specific colored lines appear on the photographic plate, unique to different substances. For example, sodium emits yellow light, while potassium emits violet light.
Absorption Spectra
Passing white light through the vapors of a substance and allowing the transmitted light to strike a prism results in dark lines in the continuous spectrum. These dark lines indicate that the substance absorbed radiations from the white light, creating an absorption spectrum.
Line Spectrum of Hydrogen
When electric discharge passes through hydrogen gas in a low-pressure tube, and the emitted light is analyzed with a spectroscope, the spectrum consists of numerous lines grouped into different series. Johannes Rydberg described these lines using a mathematical expression based on experimental observations. 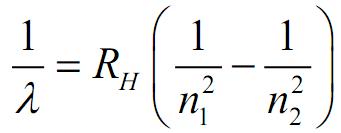 Rydberg Formula
Rydberg Formula
The above equation is valid for Hydrogen. For other elements, the equation is:
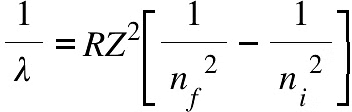 Rydberg Formula for all Elements
Rydberg Formula for all Elements
In the given formula, Z is the atomic number of the species, and RH represents a constant known as the Rydberg constant for hydrogen, RH=1.0973*107 m-1. The values of n1 and n2 are integers, and n2 is greater than n1. For a specific series, n1 remains constant, while n2 varies. For example:
- In the Lyman series, n1 is always 1, and n2 takes values such as 2, 3, 4, 5, and so on.
- For the Balmer series, n1 remains 2, while n2 can be 3, 4, 5, 6, and so forth.
- The Paschen series has n1 as 3, and n2 ranges from 4, 5, 6, 7, and onwards.
- In the Brackett series, n1 is fixed at 4, and n2 varies as 5, 6, 7, 8, and so on.
- For the Pfund series, n1 remains constant at 5, while n2 can be 6, 7, 8, 9, and beyond.

Dual Nature of Matter
Matter can exhibit either particle or wave characteristics, and numerous experiments have been conducted to prove this concept.
In 1924, de Broglie suggested that matter, similar to radiation, should display characteristics of both particles and waves. This implies that electrons, akin to photons, possess both momentum and wavelength. Using this idea, de Broglie established a relationship between the wavelength (λ) and momentum (p) of a material particle.
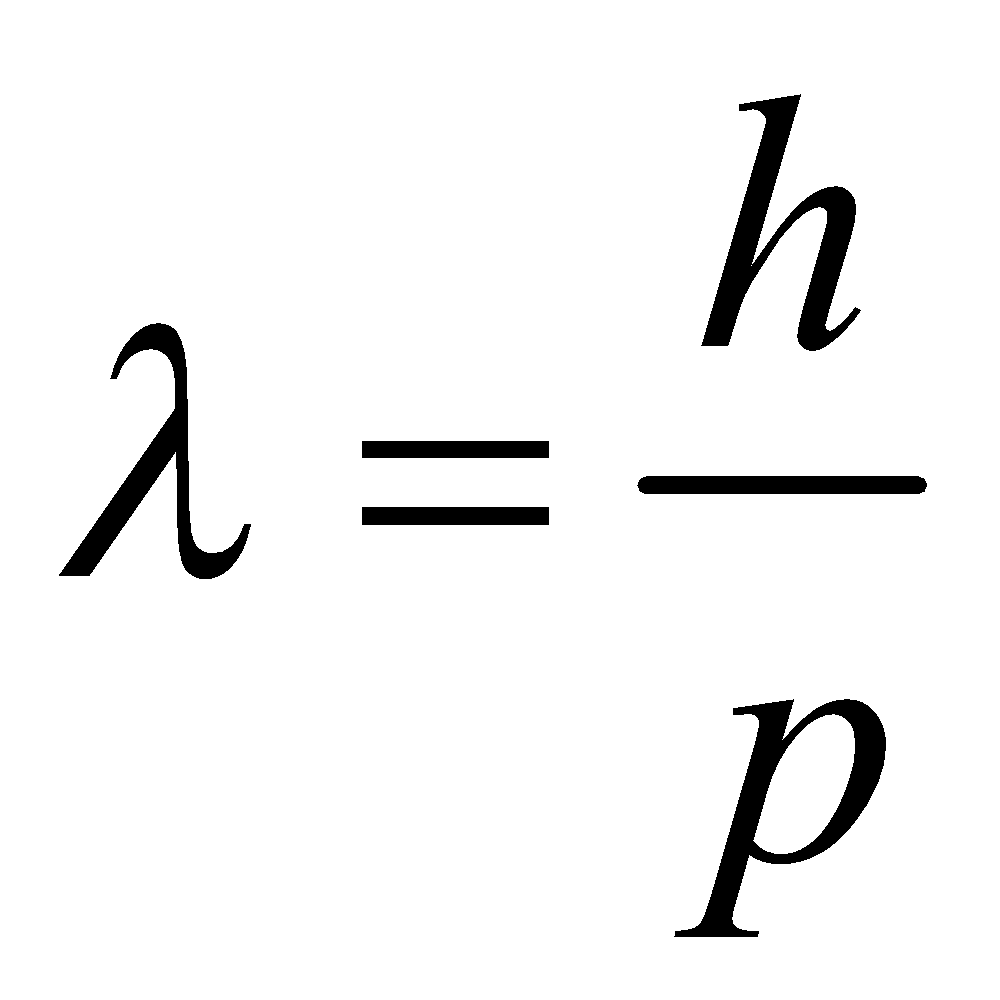 De Broglie Wavelength
De Broglie Wavelength
Heisenberg’s Uncertainty Principle
The Heisenberg Uncertainty Principle states that it is impossible to simultaneously know both the exact position and momentum of a particle with absolute precision. The more accurately one of these properties is known, the less accurately the other can be determined. In essence, there is a fundamental limit to the precision with which certain pairs of properties can be measured for a particle.
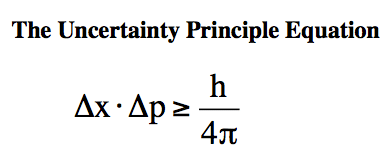 Heisenberg Uncertainty Principle Equation
Heisenberg Uncertainty Principle Equation
Drawback: Position and momentum are two such conjugate quantities that were measured accurately by Bohr (theoretically).
Electronic Configuration of an Atom
Quantum Numbers
- Principal Quantum number (n): It denotes the orbital number or shell number of the electron.
- Azimuthal Quantum numbers (l): It denotes the orbital (sub-orbit) of the electron.
- Magnetic Quantum number(m): It denotes the number of energy states in each orbit.
- Spin Quantum number(s): It denotes the direction of spin, S = -½ = Anticlockwise and ½ = Clockwise.

The electrons have to be filled in the s, p, d, and f by the following rule.
1. Aufbau’s principle:
- The Aufbau principle states that electrons occupy the lowest energy orbitals first, and higher energy orbitals are filled only after the lower energy ones are filled.
- The energy order of orbitals can be determined using the (n+l) rule, where the sum of the principal and azimuthal quantum numbers dictates the orbital's energy level.
- Lower (n+l) values mean lower orbital energies. When two orbitals have equal (n+l) values, the one with the lower principal quantum number (n) has lower energy.
- The order in which electrons fill orbitals is 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p, and so on.
2. Pauli’s exclusion principle: No two electrons can have all four quantum numbers to be the same or, if two electrons have to be placed in an energy state they should be placed with opposite spies.
3. Hund’s rule of maximum multiplicity: In the case of filling degenerate (same energy) orbitals, all the degenerate orbitals have to be singly filled first and then only pairing has to happen.
|
114 videos|263 docs|74 tests
|
FAQs on Structure of Atom Important Notes - Chemistry Class 11 - NEET
| 1. What is atomic structure and why is it important in chemistry? |  |
| 2. What are the main features of Bohr’s atomic theory? |  |
| 3. How does Planck’s quantum theory relate to atomic structure? |  |
| 4. What is the photoelectric effect and its significance in atomic physics? |  |
| 5. What is electronic configuration and why is it important? |  |

















