Class 9 Exam > Class 9 Notes > Science Class 9 > Infographics: Structure of the Atom
Infographics: Structure of the Atom | Science Class 9 PDF Download
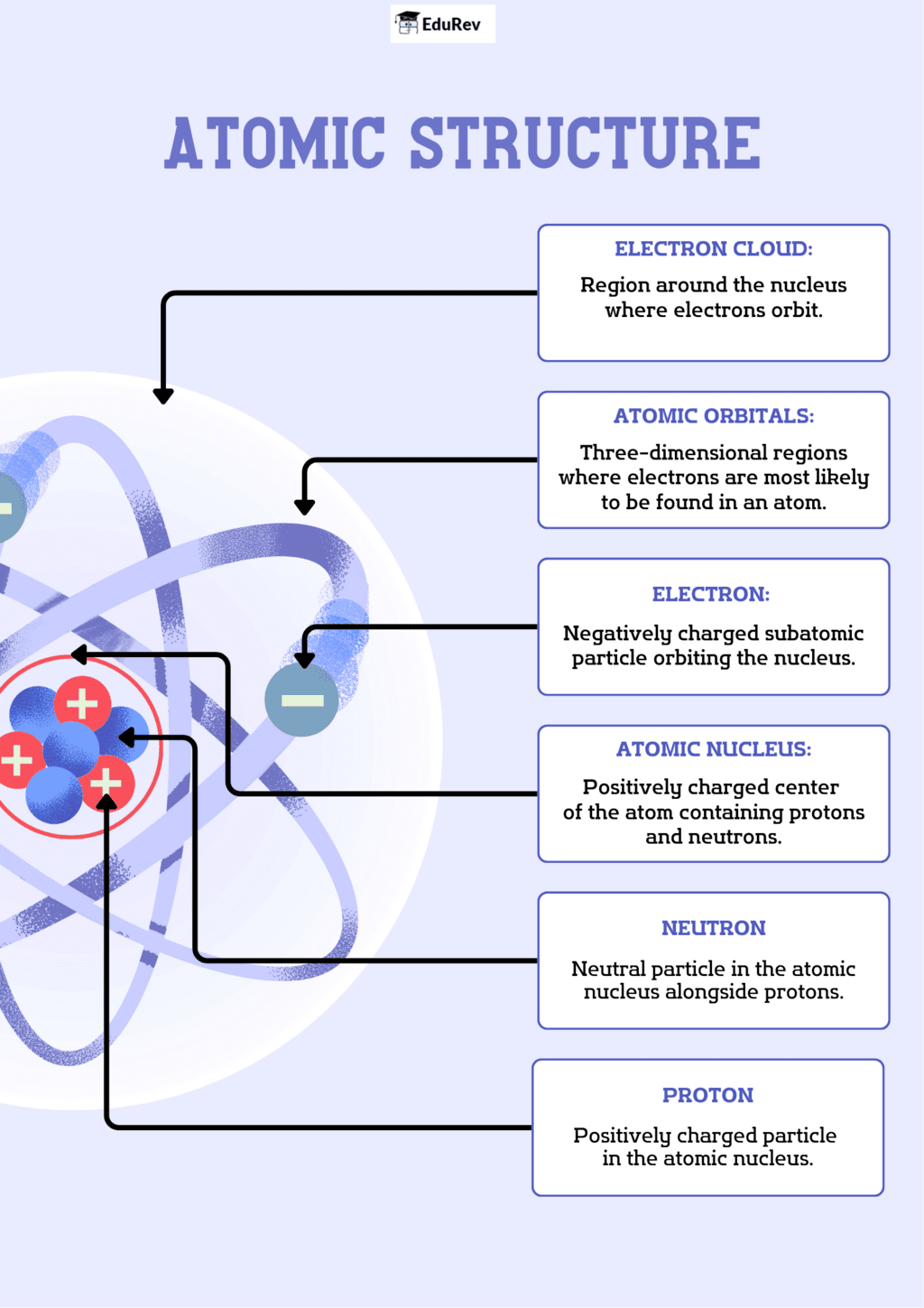
The document Infographics: Structure of the Atom | Science Class 9 is a part of the Class 9 Course Science Class 9.
All you need of Class 9 at this link: Class 9
|
84 videos|478 docs|60 tests
|
FAQs on Infographics: Structure of the Atom - Science Class 9
| 1. What are the main components of an atom? |  |
Ans. An atom is primarily composed of three subatomic particles: protons, neutrons, and electrons. Protons and neutrons are located in the nucleus at the center of the atom, while electrons orbit the nucleus in various energy levels.
| 2. How do protons and neutrons differ from electrons? |  |
Ans. Protons and neutrons are found in the nucleus of the atom and have a mass approximately 1 atomic mass unit (amu). Protons carry a positive charge, while neutrons are neutral. Electrons, on the other hand, are much lighter (approximately 1/1836 of a proton) and carry a negative charge. They exist in orbitals around the nucleus.
| 3. What is the significance of the atomic number? |  |
Ans. The atomic number of an element is defined as the number of protons in its nucleus. It determines the element's identity and its position on the periodic table. For example, an atom with an atomic number of 6 is carbon, as it has 6 protons.
| 4. How do isotopes of an element differ from one another? |  |
Ans. Isotopes are variants of a particular chemical element that have the same number of protons but different numbers of neutrons. This difference in neutron count results in different atomic masses. For example, carbon-12 and carbon-14 are both isotopes of carbon, with 6 protons but 6 and 8 neutrons, respectively.
| 5. What role do electrons play in chemical bonding? |  |
Ans. Electrons, especially those in the outermost shell (valence electrons), play a crucial role in chemical bonding. They determine how an atom interacts with others to form bonds. Atoms can gain, lose, or share electrons to achieve a full outer shell, leading to the formation of ionic or covalent bonds.
Related Searches
















