Integer Answer Type Questions: Electrochemistry | JEE Advanced | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
Integer Value Correct Type
1. All the energy released from the reaction X → Y, ΔrG° = –193 kJ mol–1 is used for oxidizing M+ as M+ → M3+ + 2e–, E° = –0.25 V
Under standard conditions, the number of moles of M+ oxidized when one mole of X is converted to Y is [F = 96500 C mol–1] (JEE Adv. 2015)
Ans: 4
Solution :
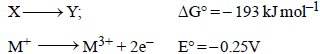
Hence DG° for oxidation will be
ΔG° = – nFE°
= –2 × 96500 × (–0.25) = 48250 J = 48.25 kJ
48.25 kJ energy oxidises one mole M+
∴ 193 kJ energy oxidises 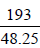 mole M+ = 4 mole M+
mole M+ = 4 mole M+
2. The molar conductivity of a solution of a weak acid HX (0.01 M) is 10 times smaller than the molar conductivity of a solution of a weak acid HY (0.10 M). If 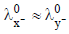 the difference in their pKa values, pKa(HX) – pKa (HY), is (consider degree of ionization of both acids to be <<1) (JEE Adv. 2015)
the difference in their pKa values, pKa(HX) – pKa (HY), is (consider degree of ionization of both acids to be <<1) (JEE Adv. 2015)
Ans: 3
Solution :
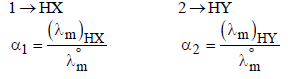
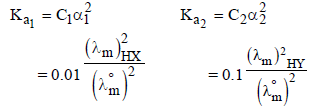
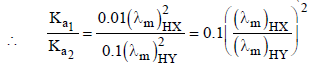
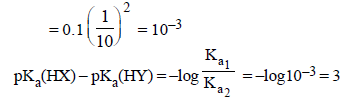
|
347 docs|185 tests
|
FAQs on Integer Answer Type Questions: Electrochemistry - JEE Advanced - 35 Years Chapter wise Previous Year Solved Papers for JEE
| 1. What is electrochemistry? |  |
| 2. What are electrodes and electrolytes in electrochemistry? |  |
| 3. What is the significance of the Nernst equation in electrochemistry? |  |
| 4. How does electroplating work in electrochemistry? |  |
| 5. What is corrosion and how does it relate to electrochemistry? |  |
|
347 docs|185 tests
|

|
Explore Courses for JEE exam
|

|

















