Integer Answer Type Questions: Thermodynamics | JEE Advanced | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
Q.1. In a constant volume calorimeter, 3.5 g of a gas with molecular weight 28 was burnt in excess oxygen at 298.0 K. The temperature of the calorimeter was found to increase from 298.0 K to 298.45 K due to the combustion process. Given that the heat capacity of the calorimeter is 2.5 kJ K–1, the numerical value for the enthalpy of combustion of the gas in kJ mol–1 is (2009 - 6M)
Ans. 9
Sol. Energy released by combustion of 3.5 g gas = 2.5 × (298.45 – 298) kJ
Energy released by 1 mole of gas 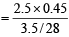 = 9 kJmol-1
= 9 kJmol-1
Q.2. One mole of an ideal gas is taken from a to b along two paths denoted by the solid and the dashed lines as shown in the graphs below. If the work done along the solid line path ws and that along the dotted line path is wd, then the integer closest to the ratio wd / ws is : (2010)
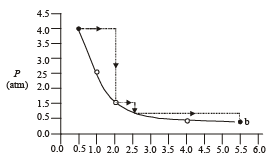
Ans. 2
Sol. 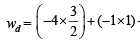
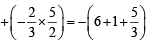
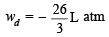
ws = – 2.303 RT log 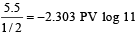
ws = – 4.606 × 1.04 = – 4.8 L atm
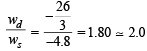
|
347 docs|185 tests
|
FAQs on Integer Answer Type Questions: Thermodynamics - JEE Advanced - 35 Years Chapter wise Previous Year Solved Papers for JEE
| 1. What is thermodynamics? |  |
| 2. What are the laws of thermodynamics? |  |
| 3. What is the difference between heat and temperature? |  |
| 4. What is an adiabatic process in thermodynamics? |  |
| 5. What is the Carnot cycle? |  |
|
347 docs|185 tests
|

|
Explore Courses for JEE exam
|

|












