Intermolecular Forces vs Thermal Interactions | Chemistry for ACT PDF Download
Everything around us is a matter which has some physical and chemical properties. Matter around us exists in different states. For a matter to occur in different states there has to be some kind of force between the atoms of this matter to hold the atoms together. These forces which are responsible for the existence of matter. Forces can be understood as some kind of fastener that holds things or bind them together.
In the case of atoms, some kind of force is needed to keep the atomic particles electrons, protons and neutrons inside the atom and hold them together for the stability of atoms. Electrons move in circular orbitals around the nucleus of an atom because of different forces that are present to provide stability.
Intermolecular Forces
Intermolecular forces can be defined as the attraction or repulsion forces that are applied to atoms and molecules when they interact with each other whether at the time of phase change or in chemical reactions. Electrostatic forces which are present between ions having different charges are not considered intermolecular forces.
These intermolecular forces are also known as Van der Waals forces when they have attractive nature. To honour a great scientist Johannes van der Waals intermolecular forces are called Van der Waals forces because he explained the concept of real gases behaviour which deviates from showing ideal behaviour under standard temperature and pressure.
As the intermolecular attraction increases, the boiling point rises. Conversely, the intermolecular forces of various substances may be evaluated by comparing their boiling temperatures. This is because the heat at the boiling point causes the intermolecular connections to break down, converting the liquid to vapour. Similarly, the melting point rises as intermolecular interactions become more intense.
Types of Intermolecular Forces
Intermolecular forces are mainly of five types as discussed below:
- Dispersion Forces or London Forces:
Dispersion Forces or London Forces can be defined as the attraction forces which are being applied between two temporary dipoles. These forces distort the electron cloud density of atoms. These forces are attractive in nature and these are named after a German physicist Fritz London and that’s why they are also known as London Forces.
These forces with an example so take two atoms X and Y which are very close to each other but are not in contact so charge distribution at any point of time if get disturbed in any of the atoms then due to the effect of London forces the charge cloud of other atoms also gets disturbed.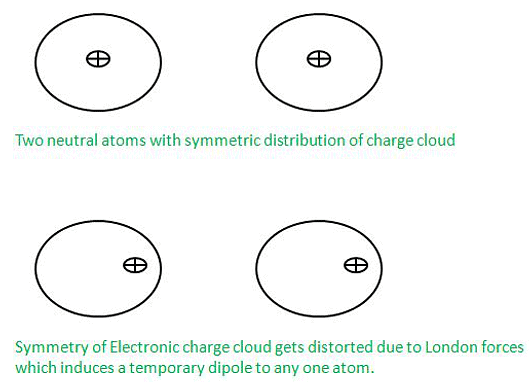
- Dipole-Dipole Forces:
Dipole-Dipole Forces can be defined as the forces which act between the molecules having permanent dipole. The dipole can be understood as a pair of opposite charges on a molecule or on a bond.
Let’s understand with an example Dipole-Dipole Forces acting between molecules of HCl as HCl molecules have permanent dipoles and due to this on Cl side, electronic charge cloud density is more than Hydrogen side.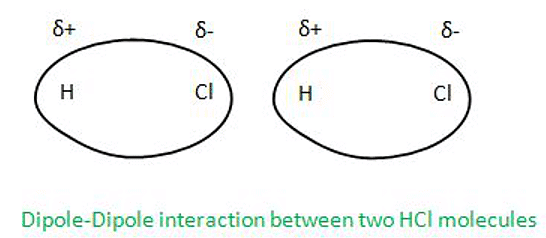
- Dipole–Induced Dipole Forces:
Dipole–Induced Dipole Forces can be defined as the attraction forces which are being applied between two molecules in which one has a permanent dipole and the other does not have a permanent dipole. Hence the molecule with permanent dipole induces the dipole to the other molecule which is electrically neutral and hence distorts the electronic charge cloud.
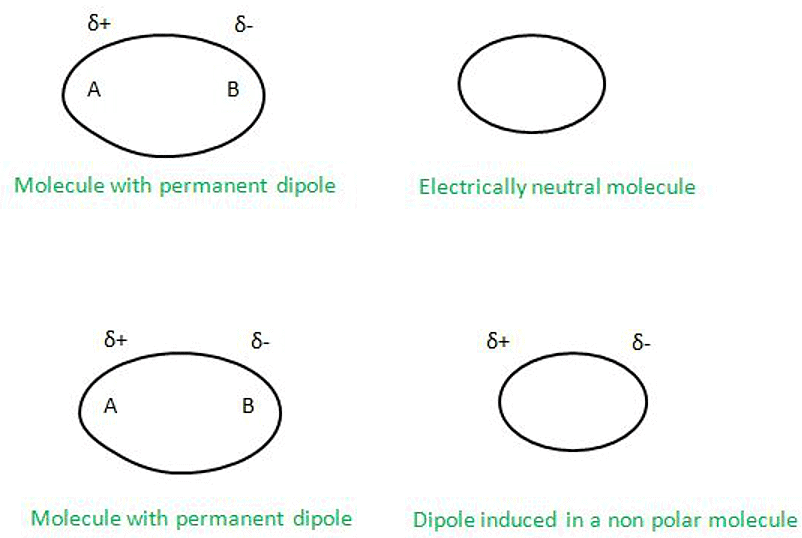
- Ion-Dipole Interactions
This is the attraction between a polar molecule and an ion (cation or anion). When NaCl is dissolved in water, the polar water molecules are attracted to the Na+ and Cl– ions (a process known as ion hydration). The strength of this contact is determined by the polar molecule’s dipole moment and size, as well as the charge and size of the ion. Because the cation has a higher charge density than the same-charged anion, this contact is generally stronger with the cation. Furthermore, because CCl4 is non-polar, it cannot interact with the cations Na+ and Cl–. As a result, NaCl is insoluble in CCl4.
Because the charge of any ion is substantially more than the charge of a dipole moment, ion-dipole attraction forces are stronger than dipole-dipole interactions. - Ion-Induced Dipole Interactions
The presence of an ion near a non-polar molecule can cause it to become polarised, causing it to become an induced dipole. Ion-induced dipole interactions are the interactions between them. The intensity of these interactions is determined by the ion’s charge and the situation in which the non-polar molecule becomes polarised. An anion polarises the molecule via repulsion, whereas a cation polarises it through the attraction of the electron cloud.
Haemoglobin, for example, is present in red blood cells (RBC). It has a Fe2+ ion in its core, which attracts the O2 ion through the ion-induced dipole force.
Thermal Energy
The energy contained inside a system that is accountable for its temperature is referred to as thermal energy. The transfer of thermal energy is referred to as heat. Thermodynamics is a discipline of physics that studies how heat is transmitted across systems and how work is done in the process (see the first law of thermodynamics).
Thermal Energy can be defined as the energy which arises due to the motion of atoms and molecules of any system. The motion of particles of a system is known as thermal motion which gives rise to thermal energy and this thermal energy varies directly with the temperature.
The energy of a single particle due to motion is known as kinetic energy but the average energy of all particles gives the measure of thermal energy.
Difference between Thermal Energy and Intermolecular Forces
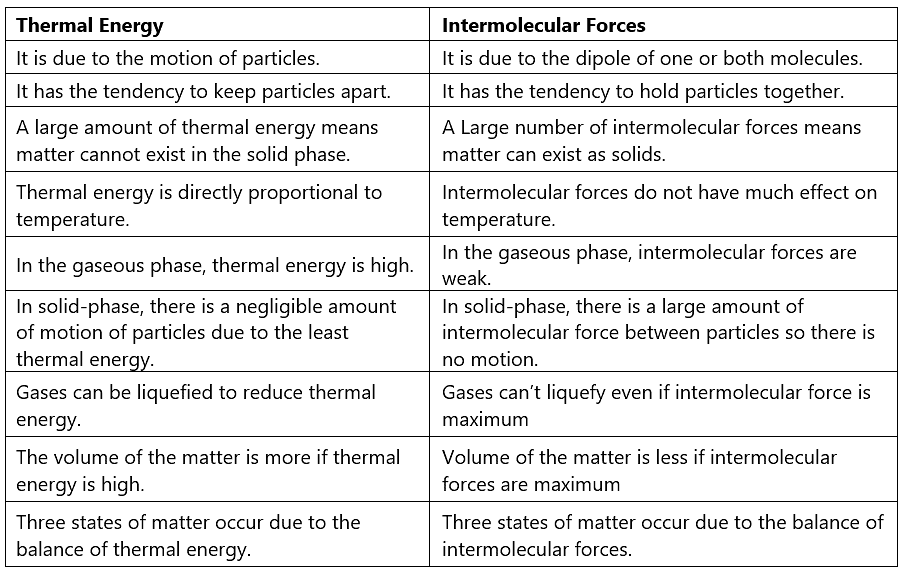
|
110 videos|130 docs|117 tests
|




















