Grade 10 Exam > Grade 10 Notes > Chemistry for Grade 10 > Introduction to Carbonates
Introduction to Carbonates | Chemistry for Grade 10 PDF Download
Test for Carbonates
- Carbonates all contain the carbonate ion, CO32-
- The test for this ion involves adding dilute acid and testing the gas released
- If a carbonate compound is present then effervescence should be seen and the gas produced is CO2 which forms a white precipitate of calcium carbonate when bubbled through limewater:
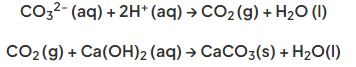
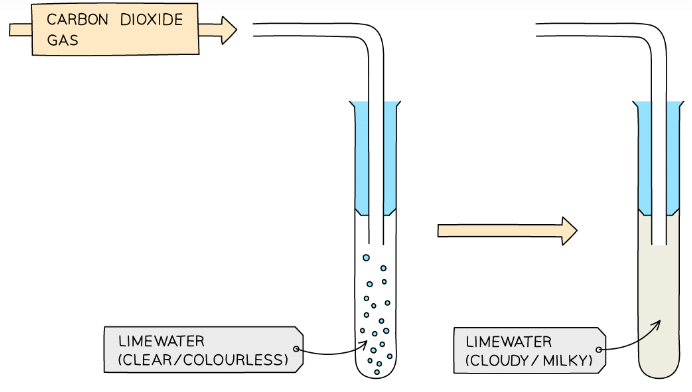 Limewater turns milky in the presence of CO2 caused by formation of insoluble calcium carbonate
Limewater turns milky in the presence of CO2 caused by formation of insoluble calcium carbonate
Exam Tip
You’ll need to connect the test tube of the suspected ion to the test tube of limewater quickly so none of the CO2 escapes.
The document Introduction to Carbonates | Chemistry for Grade 10 is a part of the Grade 10 Course Chemistry for Grade 10.
All you need of Grade 10 at this link: Grade 10
|
75 videos|131 docs|24 tests
|
Related Searches















