Isomerism In Coordination Compounds-1 - Coordination Chemistry | Inorganic Chemistry PDF Download
ISOMERISM IN COORDINATION COMPOUNDS
Isomers are the compounds with the same chemical formula but different arrangement of their constituent atoms and the phenomenon is called isomerism. Isomerism in Coordination compounds is classified into two types: structural or constitutional isomerism and stereoisomerism.
Structural Isomerism : Isomers that have different bonding pattern between its atoms are called structural isomers.
(i) Ionization Isomerism: Ionization isomers results from the exchange an anionic ligands within the coordination sphere with the counter ion outside the sphere. For example, violet [Co(NH3)5Br]SO4 and red [Co(NH3)5(SO4)]Br are ionization isomers.
These isomers can be easily differentiated by qualitative test for sulphate and bromide. For example, in aqueous solution [Co(NH3)5Br]SO4 gives white ppt. of BaSO4 on reaction with BaCl2 whereas it does not give any ppt. with AgNO3. This indicates that SO42– is present as counter ion outside the coordination sphere.
On the other hand, [Co(NH3)5(SO4)]Br gives light yellow ppt. of AgBr with AgNO3 and it does not give any ppt. with BaCl2. This indicates that Br- is present outside the coordination sphere.
These isomers can also be differentiated by IR spectroscopy. Some other examples of ionization isomerism are:
a. [Co(NH3)5Cl]SO4 and [Co(NH3)5(SO4)]Cl
b. [Co(NH3)5(NO3)]SO4 and [Co(NH3)5(SO4)]NO3
c. [Pt(NH3)4Cl2]Br2 and [Pt(NH3)4Br2]Cl2
d. [Co(en)2Cl(NO2)]SCN and [Co(en)2Cl(SCN)]NO2 and [Co(en)2(NO2)(SCN)]Cl
(ii) Hydration Isomerism: When there is exchange between the water molecules in coordination sphere and the water of hydration, then the resulting isomers are called hydrate isomers. For example, hydrate isomers of the compound having formula CrCl3.6H2O are:
[Cr(H2O)6]Cl3 (violet)
[Cr(H2O)5Cl](H2O)Cl2 (pale green)
[Cr(H2O)4Cl2](2H2O)Cl (dark green)
These isomers have different properties: a. These isomers have different colours.
b. The complexes can be distinguished by precipitation of the free chloride ion using aqueous silver nitrate. For example, when these isomers react with AgNO3 solution, the y give white ppt. of AgCl corresponding to One, two and three mole of chloride ion respectively.
c. On treating these complexes with conc. H2SO4 the water of hydration is lost.
(iii) Linkage Isomerism: Linkage isomers may arise when one or more of the ligands can coordinate to the metal ion in more than one way (ambidentate ligand), e.g. in [SCN]-, both the N and S atoms are potential donor sites.
[Co(NH3 )5 (SCN)]Cl2 and [Co( NH3 )5 ( NCS)]Cl2
Linkage Isomers
Other examples of linkage isomers are:
a. [Co(NH3)5(NO2)]2+ and [Co(NH3)5(ONO)]2+
b. [Co(en)2(NO2)2]+ and [Co(en)2(ONO)2]+
c. [Pd(PPh3)2(SCN)2] and [Pd(PPh3),(NCS)2]
(iv) Coordination Isomerism: Coordination isomers are possible only for salts in which both cation and anion are complex ions. These isomers arise from interchange of ligands between the two metal centres. For example,
a. [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]
b. [Co(NH3)6][Co(NO2)6] and [Co(NH3)4(NO2)][Co(NH3)2(NO2)4]
c. [Pt (NH3)4 ][PtCl6] and [Pt (NH3)4 Cl2 ][PtCl4 ]
(v) Coordination Position Isomerism: This isomerism exists in bridging complexes. In bridging complexes the exchange of non-bridging ligands between two metal cations give rise to coordination position isomerism.
For example,
(vi) Ligand Isomerism: This isomerism takes place in complexes in which ligand itself exists in two or more isomeric form. For Example,
(vii) Polymerization Isomerism: These isomers have same empirical formula instead of molecular formula. All these isomers have same ratio of metal atoms and the ligand in them. For example, Coordination polymers of Pt2+ ion
Stereoisomerism Stereoisomers
are isomers that differ only in spatial arrangement of ligands coordinated to metal cation/atom.
These are of two types:
(a) Geometrical or Cis/Trans Isomerism
(b) Optical Isomerism
(a) Geometrical Isomerism:
Stereoisomers in which the orientations or relative positions of the ligands around the metal cation is different are called geometrical isomers and this phenomenon is geometrical isomerism. For example,
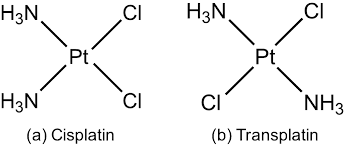
These isomers are also called cis-trans isomers.
Geometrical isomers cannot be interconverted without breaking of M-L bonds. Geometrical isomerism is most common in complexes having coordination no. 4 and 6. The complexes which exhibit coordination no. 2 and 3 do not exhibit geometrical isomerism. Now, we will discuss geometrical isomerism in each type of complexes.
Tetrahedral Complexes: Tetrahedral complexes do not exhibit geometrical isomerism whether all the ligands are same or different because all the ligands in this geometry are at adjacent position relative to each other.
Square Planar Complexes: These are of different types depending upon the nature and type of ligands. Complexes can be either ionic of neutral. The charge does not effect on their property of exhibiting geometrical isomers. For convenience, we will take neutral complexes.
- [Ma4]n±, [Ma3b]n±, [M(AA)2]n±, [M(AA)ab]n± and [M(AA)a2]n± type square planar complexes do not exhibit geometrical isomerism because all the possible spatial arrangement of the ligands around the metal cation is the same.
- [Ma2b2]n± type complexes: These types of complexes exist as cis and trans isomers.
For example, [Pt(NH3)2Cl2], [Pt(py)2Cl2] etc.
- [Ma2bc]n±: These types of complexes also exist as cis and trans iso mers.
Examples are, [Pt(NH3)2(NO2)Cl], [Pt(NH3)2(Py)Cl]+, [Pt(Py)2(NH3)Cl] etc.
- [Mabcd]n±: Three geometrical isomers are possible for these type of complexes.
Examples are, [Pt(Py)(NH3)ClBr], [Pt(C2H4 )(NH3 )ClBr] and [Pt(Py)(NH3)(NH2OH)(NO2 )]+
- [M(AB)2]n±: Two geometrical isomers are possible.
for Examples :
- Bridged Binuclear Square Planar Complexes of [M2a2b4] type: These typ es of complexes can exist in three isomeric form namely, cis, trans and unsymmetric.
For example [Pt (PEt3) Cl2]2
- Square planar complexes with symmetric bidentate ligands carrying one or more substituents can exist as geometrical isomers. For example, [Pt(pn)2]2+ exist in cis- and trans- isomeric forms in which methyl groups are cis- and trans- respectively with respect to the median plane of the ring atoms.
Another example is that having bidentate ligands with two methyl substituents as shown below:
Octahedral Complexes: In an octahedral complex a metal cation will present in the centre of an octahedron and the six ligands occupy the six corners numbered from 1 to 6 as shown below:
In cis isomers the two ligands occupy the corners of octahedral adjacent to each other. In cis isomers the same ligands occup y either of the positions (1, 2), (1, 3), (1, 5), (1, 6), (2, 3), (2, 4), (2, 6), (3, 4), (3, 5), (4, 5), (4, 6), (5, 2), (6, 3), or (6,5).
In trans isomers these ligands are lying opposite to one another on a straight line which passes through the centre of the octahedron. In trans isomer the two ligand under consideration will occupy either of the positions (1, 6), (2, 4) and (3, 6).
[Ma6]n±, [Ma5b]n± and [M(AA)3]n± (where AA is a symmetric bidentate ligand) complexes will not have geometrical isomers because all the corners of regular tetrahedron are equivalent.
The following type of octahedral complexes exhibit geometrical isomerism:
[Ma4b2]n±: Two geometrical isomers.
For examples: [Co(NH3 )4Cl2 ]+ , [Co(NH3 )4 (NO2 )]+
[Ma4bc]n±: Two isomers (cis and trans).
For examples: [Co(NH3)4 (H2O)Cl] , [Co(NH3)4 (Py)Cl)]2+
|
50 videos|92 docs|41 tests
|
FAQs on Isomerism In Coordination Compounds-1 - Coordination Chemistry - Inorganic Chemistry
| 1. What is isomerism in coordination compounds? |  |
| 2. What are the different types of isomerism in coordination compounds? |  |
| 3. How does geometric isomerism occur in coordination compounds? |  |
| 4. What is the significance of isomerism in coordination compounds? |  |
| 5. How is isomerism in coordination compounds determined experimentally? |  |

















